Home /
Expert Answers /
Chemistry /
balance-each-of-the-following-oxidation-reduction-reactions-by-using-the-oxidation-states-method-pa748
(Solved): Balance each of the following oxidation-reduction reactions by using the oxidation states method. ( ...
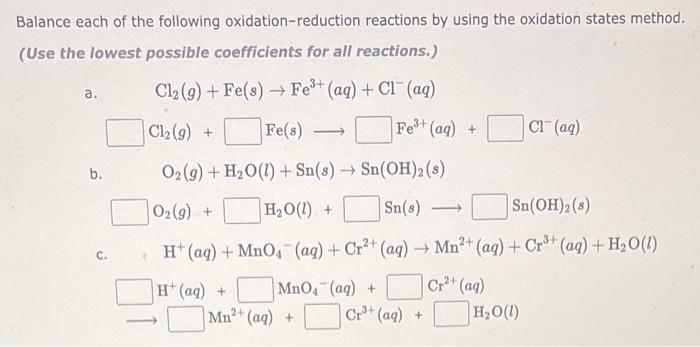
Balance each of the following oxidation-reduction reactions by using the oxidation states method. (Use the lowest possible coefficients for all reactions.) a. \[ \begin{array}{l} \mathrm{Cl}_{2}(g)+\mathrm{Fe}(s) \rightarrow \mathrm{Fe}^{3+}(a q)+\mathrm{Cl}^{-}(a q) \\ \mathrm{Cl}_{2}(g)+\quad \mathrm{Fe}(s) \longrightarrow \mathrm{Fe}^{3+}(a q)+\mathrm{Cl}^{-}(a q) \end{array} \] b. \( \mathrm{O}_{2}(g)+\mathrm{H}_{2} \mathrm{O}(l)+\mathrm{Sn}(s) \rightarrow \mathrm{Sn}(\mathrm{OH})_{2}(s) \) \[ \mathrm{O}_{2}(g)+\quad \mathrm{H}_{2} \mathrm{O}(l)+\quad \mathrm{Sn}(s) \longrightarrow \quad \mathrm{Sn}(\mathrm{OH})_{2}(s) \] c. \( \mathrm{H}^{+}(a q)+\mathrm{MnO}_{4}^{-}(a q)+\mathrm{Cr}^{2+}(a q) \rightarrow \mathrm{Mn}^{2+}(a q)+\mathrm{Cr}^{3+}(a q)+\mathrm{H}_{2} \mathrm{O}(l) \)