Home /
Expert Answers /
Chemical Engineering /
b-using-the-latimer-diagram-for-re-in-a-basic-solution-find-the-standard-reduction-potential-for-pa792
(Solved): (b) Using the Latimer Diagram for Re in a basic solution, find the standard reduction potential for ...
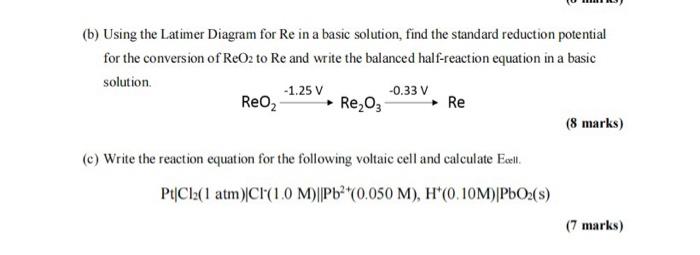
(b) Using the Latimer Diagram for Re in a basic solution, find the standard reduction potential for the conversion of \( \mathrm{ReO}_{2} \) to Re and write the balanced half-reaction equation in a basic solution. \[ \mathrm{ReO}_{2} \stackrel{-1.25 \mathrm{~V}}{\longrightarrow} \mathrm{Re}_{2} \mathrm{O}_{3} \stackrel{-0.33 \mathrm{~V}}{\longrightarrow} \mathrm{Re} \] (8 marks) (c) Write the reaction equation for the following voltaic cell and calculate Exell. \[ \mathrm{Pt}\left|\mathrm{Cl}_{2}(1 \mathrm{~atm})\right| \mathrm{Cl}^{-}(1.0 \mathrm{M})|| \mathrm{Pb}^{2+}(0.050 \mathrm{M}), \mathrm{H}^{+}(0.10 \mathrm{M}) \mid \mathrm{PbO}_{2}(\mathrm{~s}) \] (7 marks)
Expert Answer
b. The latimer diagram represents the electrochemical equilibria for compounds that exist in different oxidation states.