Home /
Expert Answers /
Chemical Engineering /
b-a-galvanic-cell-consists-of-a-pb-electrode-in-a-1-0mpb-no3-2-solution-and-pt-electrode-in-pa971
(Solved): b) A galvanic cell consists of a Pb electrode in a 1.0MPb(NO3)2 solution and Pt electrode in ...
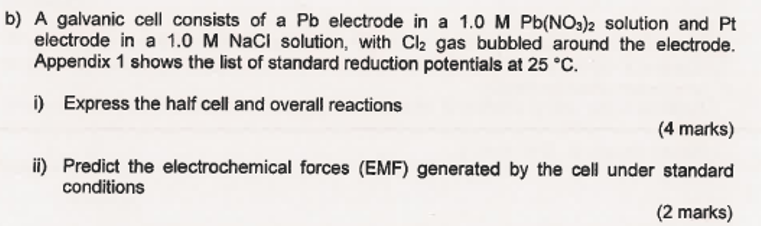
b) A galvanic cell consists of a electrode in a solution and electrode in a solution, with gas bubbled around the electrode. Appendix 1 shows the list of standard reduction potentials at . i) Express the half cell and overall reactions (4 marks) ii) Predict the electrochemical forces (EMF) generated by the cell under standard conditions (2 marks)
Expert Answer
b) Solution:-Given data:- A galvanic cell consists of a Pb electrode in a 1.0 M Pb(NO3)2 solution and Pt electrode in a 1.0 M NaCl solution, with Cl?