Home /
Expert Answers /
Chemistry /
at-a-certain-temperature-the-equilibrium-constant-for-the-chemical-reaction-shown-is-5-88-time-pa989
(Solved): At a certain temperature, the equilibrium constant for the chemical reaction shown is \( 5.88 \time ...
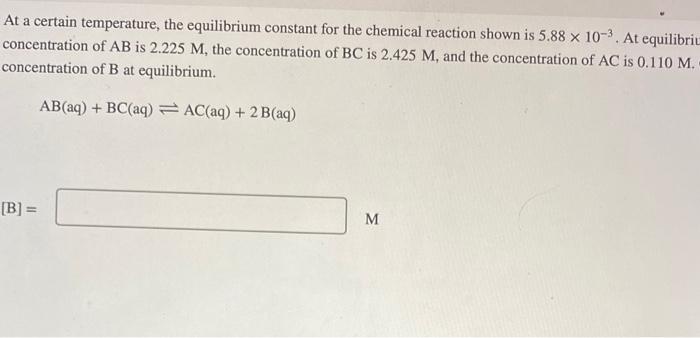
At a certain temperature, the equilibrium constant for the chemical reaction shown is \( 5.88 \times 10^{-3} \). At equilibrit concentration of \( \mathrm{AB} \) is \( 2.225 \mathrm{M} \), the concentration of \( \mathrm{BC} \) is \( 2.425 \mathrm{M} \), and the concentration of \( \mathrm{AC} \) is \( 0.110 \mathrm{M} \). concentration of \( \mathrm{B} \) at equilibrium. \[ \mathrm{AB}(\mathrm{aq})+\mathrm{BC}(\mathrm{aq}) \rightleftharpoons \mathrm{AC}(\mathrm{aq})+2 \mathrm{~B}(\mathrm{aq}) \] \[ \text { [B] }= \] \( \mathrm{M} \)
Expert Answer
Equilibrium constant is the ratio of pro