Home /
Expert Answers /
Chemical Engineering /
assume-100gmol-of-synthesis-gas-q-3-a-synthesis-gas-at-500-deg-mathrm-c-that-analyzes-pa418
(Solved): Assume 100gmol of synthesis gas. Q.3. A synthesis gas at 500 deg \( \mathrm{C} \) that analyzes \( ...
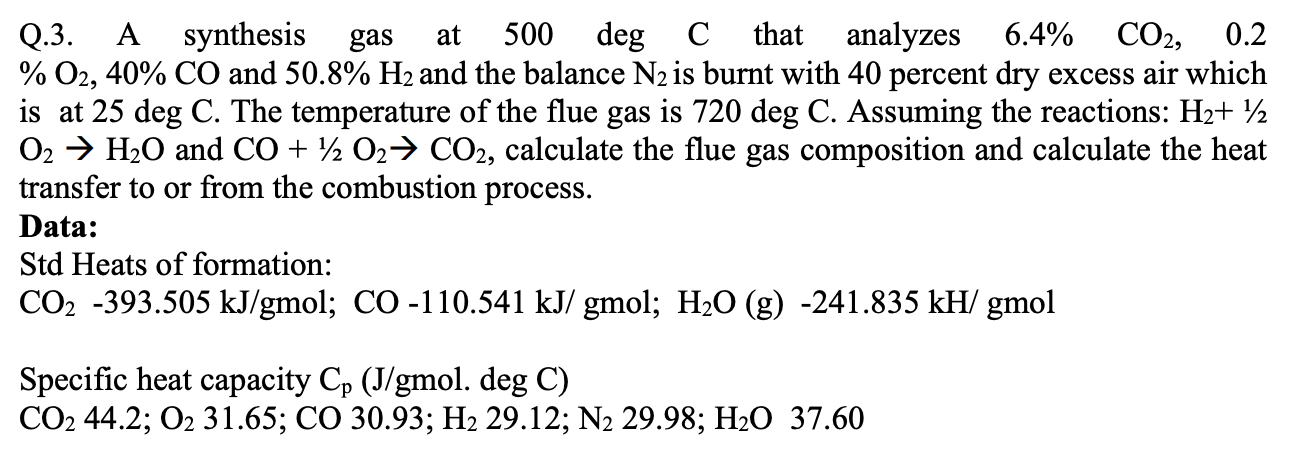
Q.3. A synthesis gas at 500 deg \( \mathrm{C} \) that analyzes \( 6.4 \% \mathrm{CO}_{2}, 0.2 \) \( \% \mathrm{O}_{2}, 40 \% \mathrm{CO} \) and \( 50.8 \% \mathrm{H}_{2} \) and the balance \( \mathrm{N}_{2} \) is burnt with 40 percent dry excess air which is at \( 25 \mathrm{deg} \mathrm{C} \). The temperature of the flue gas is \( 720 \mathrm{deg} \) C. Assuming the reactions: \( \mathrm{H}_{2}+1 / 2 \) \( \mathrm{O}_{2} \rightarrow \mathrm{H}_{2} \mathrm{O} \) and \( \mathrm{CO}+1 / 2 \mathrm{O}_{2} \rightarrow \mathrm{CO}_{2} \), calculate the flue gas composition and calculate the heat transfer to or from the combustion process. Data: Std Heats of formation: \( \mathrm{CO}_{2}-393.505 \mathrm{~kJ} / \mathrm{gmol} ; \mathrm{CO}-110.541 \mathrm{~kJ} / \mathrm{gmol} ; \mathrm{H}_{2} \mathrm{O}(\mathrm{g})-241.835 \mathrm{kH} / \mathrm{gmol} \) Specific heat capacity \( \mathrm{C}_{\mathrm{p}}(\mathrm{J} / \mathrm{gmol} \). deg \( \mathrm{C}) \) \( \mathrm{CO}_{2} 44.2 ; \mathrm{O}_{2} 31.65 ; \mathrm{CO} 30.93 ; \mathrm{H}_{2} 29.12 ; \mathrm{N}_{2} 29.98 ; \mathrm{H}_{2} \mathrm{O} 37.60 \)