Home /
Expert Answers /
Chemistry /
as-solute-is-dissolved-in-a-solvent-the-vapor-pressure-of-the-solution-changes-according-to-raoul-pa812
(Solved): As solute is dissolved in a solvent, the vapor pressure of the solution changes according to Raoul ...
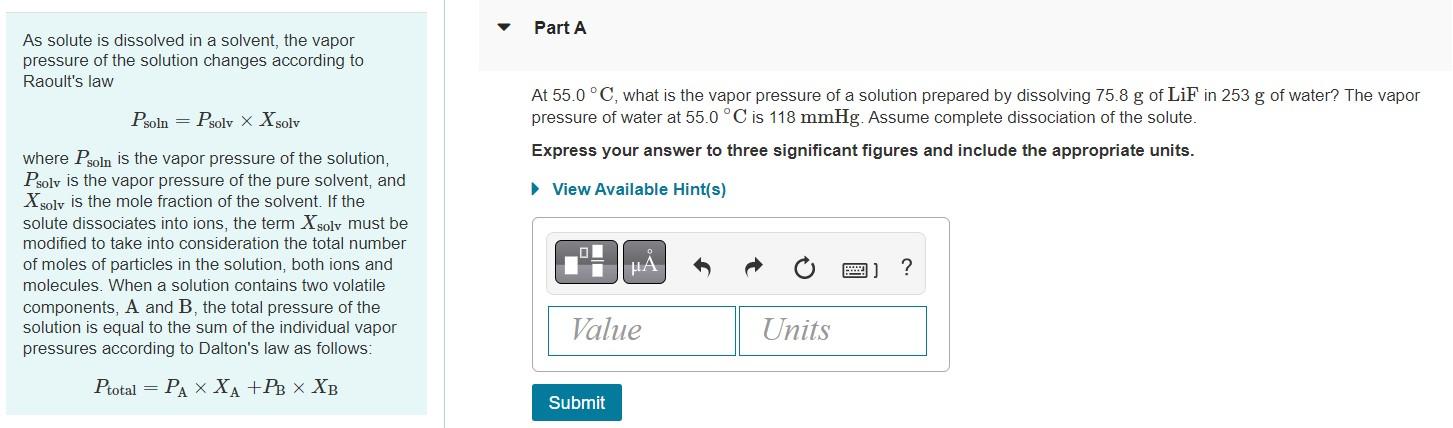
As solute is dissolved in a solvent, the vapor pressure of the solution changes according to Raoult's law \[ P_{\text {soln }}=P_{\text {solv }} \times X_{\text {solv }} \]
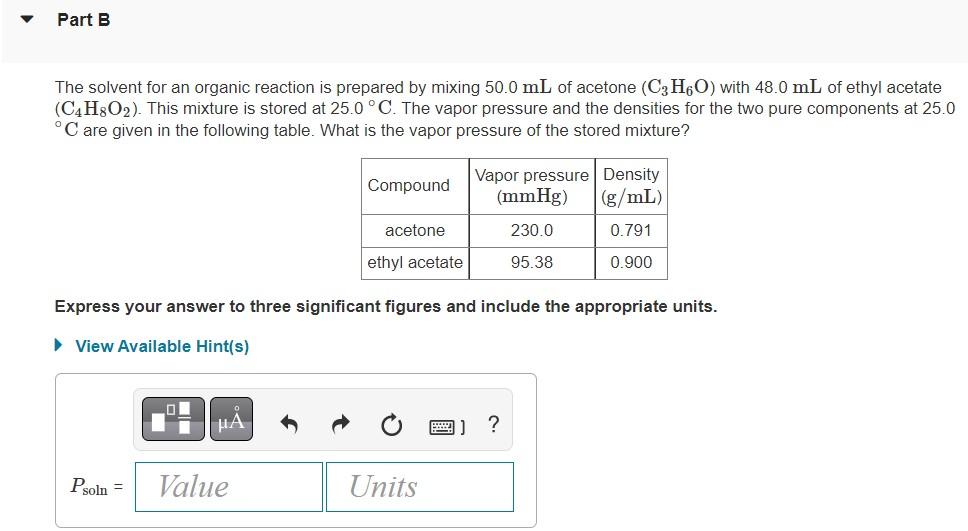
The solvent for an organic reaction is prepared by mixing \( 50.0 \mathrm{~mL} \) of acetone \( \left(\mathrm{C}_{3} \mathrm{H}_{6} \mathrm{O}\right) \) with \( 48.0 \mathrm{~mL} \) of ethyl acetate \( \left(\mathrm{C}_{4} \mathrm{H}_{8} \mathrm{O}_{2}\right) \). This mixture is stored at \( 25.0^{\circ} \mathrm{C} \). The vapor pressure and the densities for the two pure components at \( 25.0 \) \( { }^{\circ} \mathrm{C} \) are given in the following table. What is the vapor pressure of the stored mixture? Express your answer to three significant figures and include the appropriate units.