Home /
Expert Answers /
Chemistry /
arenediazoniums-can-undergo-electrophilic-aromatic-substitutions-with-a-wide-variety-of-activated-a-pa573
(Solved): Arenediazoniums can undergo electrophilic aromatic substitutions with a wide variety of activated a ...

Arenediazoniums can undergo electrophilic aromatic substitutions with a wide variety of activated aromatic compounds to yield new azo dyes. This chemistry is also seen in the Sandmeyer reaction. Consider the reaction sequence below, which forms an arenediazonium in situ using p-nitroaniline as one of the starting materials, and answer the questions that follow.
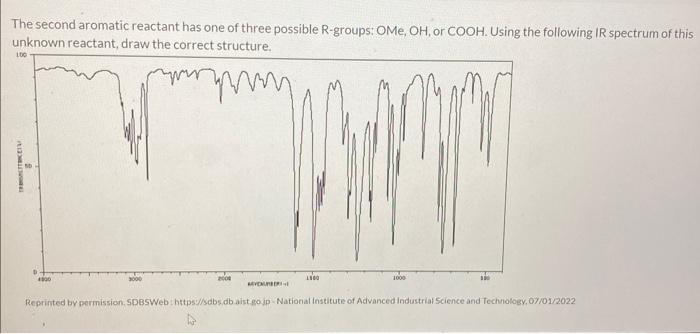
The second aromatic reactant has one of three possible R-groups: \( \mathrm{OMe}, \mathrm{OH} \), or \( \mathrm{COOH} \). Using the following IR spectrum of this unknown reactant, draw the correct structure. Reprinted by permission. SDBSWeb : https.u/sdbs db aist go jp. National institute of Advanced industrial 5 cience and Techuiologv, 07/0 12022
Use the identified second reactant structure from above to draw the azo coupling product (azo dye) which results from this reaction.
Expert Answer
The second rectant used in the above reaction is Anisole on analysing of ir