Home /
Expert Answers /
Advanced Physics /
an-atomic-line-spectra-of-an-unknown-substance-taken-in-the-lab-is-shown-to-the-right-using-the-co-pa412
(Solved): An atomic line spectra of an unknown substance taken in the lab is shown to the right. Using the co ...
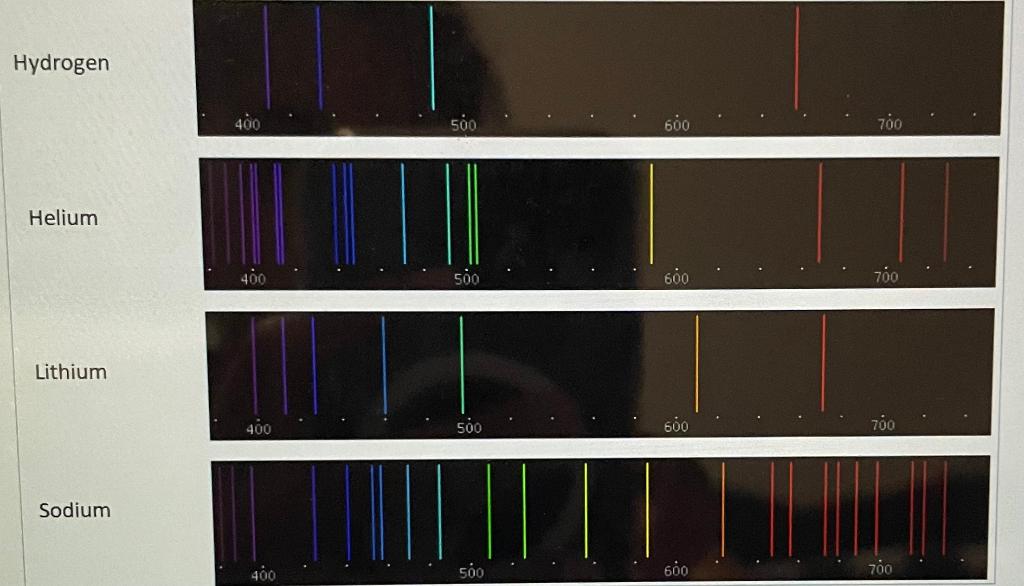
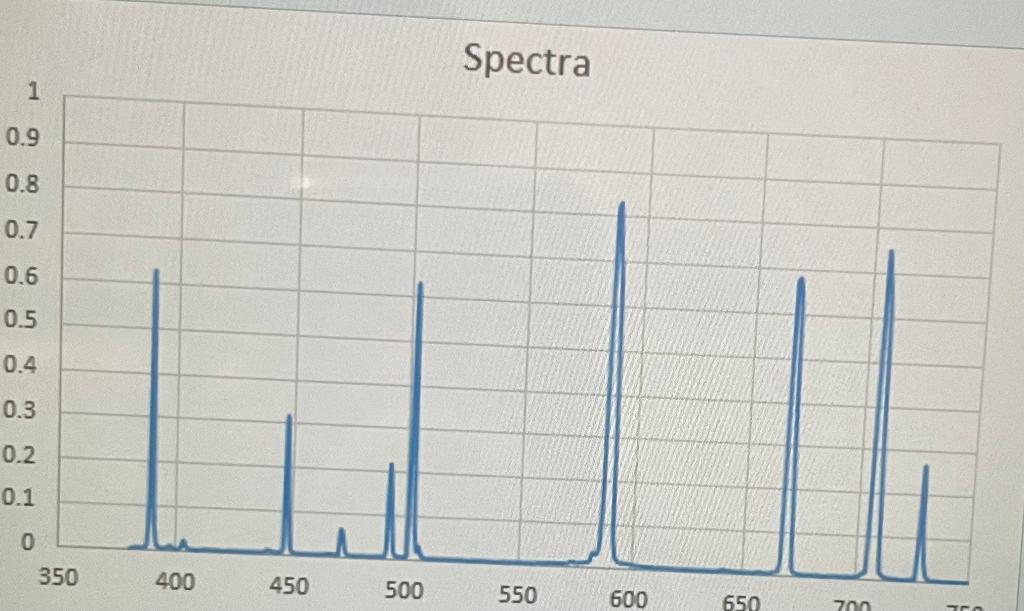
An atomic line spectra of an unknown substance taken in the lab is shown to the right.
Using the color reference below, determine the unknown substance.
The unknown substance is helium
Photons emitted in the visible spectra of Helium which have the highest energy will have a wavelength of?
388 nm
447 nm
588 nm
706 nm
728 nm
In the spectra of Helium, more photons are emitted with a wavelength of ________ than any other wavelength
388 nm
447 nm
588 nm
706 nm
728 nm
ANSWER 3RD QUESTION!!!!!!!!!!!!!!!!!!!!!
Lithium Sodium
Spectra
Expert Answer
using the energy formula E = (hc/ ),