(Solved): Among the substances that react with oxygen and that have been considered as potential rocket fuels ...
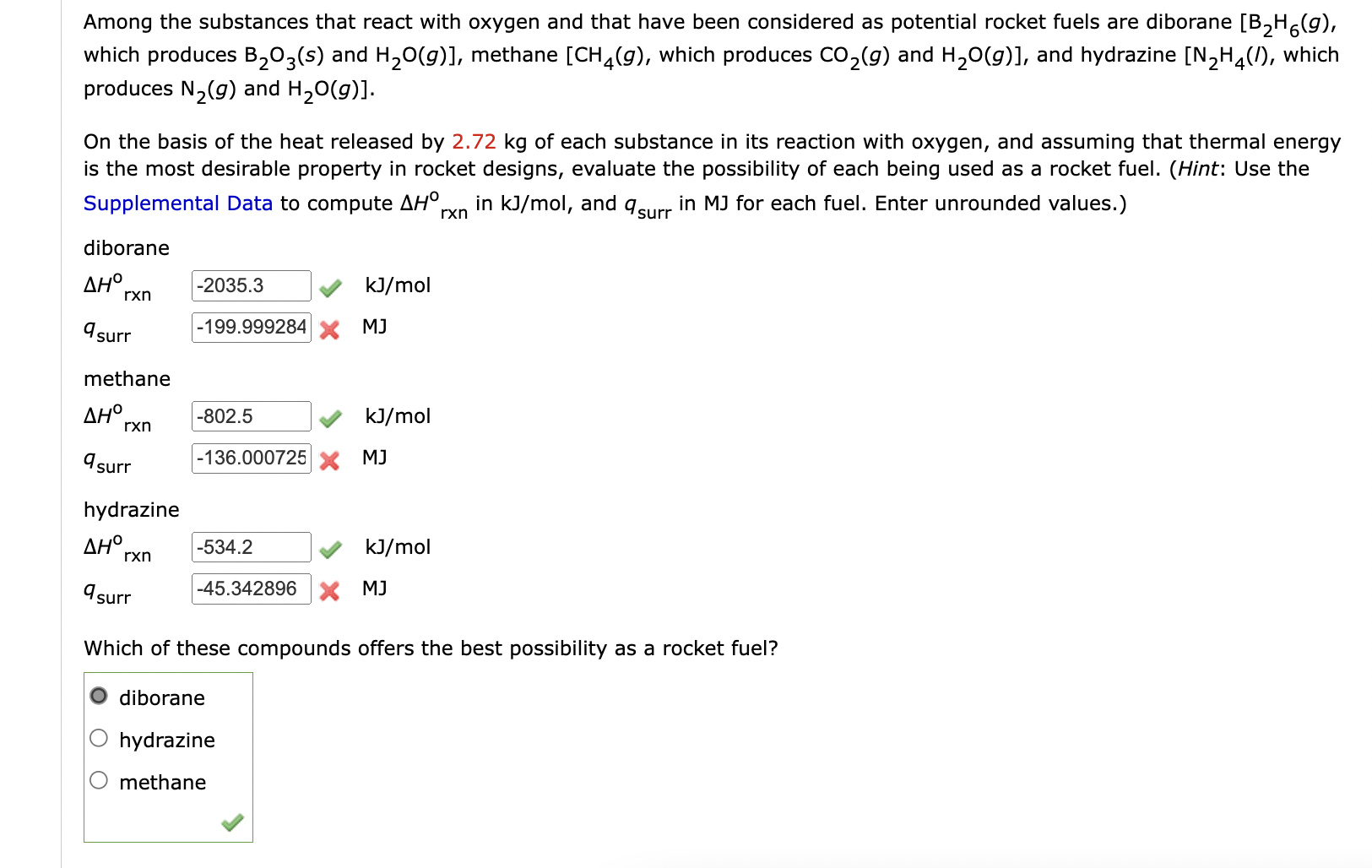
Among the substances that react with oxygen and that have been considered as potential rocket fuels are diborane [B2H6(g), which produces B2O3(s) and H2O(g)], methane [CH4(g), which produces CO2(g) and H2O(g)], and hydrazine [N2H4(l), which produces N2(g) and H2O(g)]. On the basis of the heat released by 2.72 kg of each substance in its reaction with oxygen, and assuming that thermal energy is the most desirable property in rocket designs, evaluate the possibility of each being used as a rocket fuel. (Hint: Use the Supplemental Data to compute \Delta Horxn in kJ/mol, and qsurr in MJ for each fuel. Enter unrounded values.) Among the substances that react with oxygen and that have been considered as potential rocket fuels are diborane , which produces
B_(2)O_(3)(s)and
{:H_(2)O(g)], methane , which produces
CO_(2)(g)and
{:H_(2)O(g)], and hydrazine , which produces
N_(2)(g)and
H_(2)O(g)]. On the basis of the heat released by
2.72kgof each substance in its reaction with oxygen, and assuming that thermal energy is the most desirable property in rocket designs, evaluate the possibility of each being used as a rocket fuel. (Hint: Use the Supplemental Data to compute
\Delta H\deg _(r\times n)in
k(J)/(m)ol, and
q_(surr )in
MJfor each fuel. Enter unrounded values.) diborane
\Delta H_(rxn )^(o)
k(J)/(m)ol
q_(surr )MJ methane
\Delta H_(rxn )^(0)
k(J)/(m)ol
q_(surr )MJ hydrazine
\Delta H_(rxn )\deg
q_(surr )
k(J)/(m)olWhich of these compounds offers the best possibility as a rocket fuel? diborane hydrazine methane