Home /
Expert Answers /
Chemistry /
amides-can-undergo-a-two-step-hydrolysis-process-2-hclh2o1-naoh-draw-the-final-p-pa584
Expert Answer
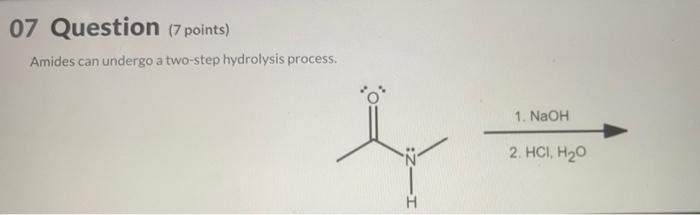
The hydrolysis of amide is as follows: step-1: Nucleophile attack to the electrophilic center