Home /
Expert Answers /
Chemistry /
alkenes-are-more-reactive-than-benzene-and-undergo-addition-reactions-such-as-decolourizing-bromi-pa514
(Solved): Alkenes are more reactive than benzene and undergo addition reactions, such as decolourizing bromi ...
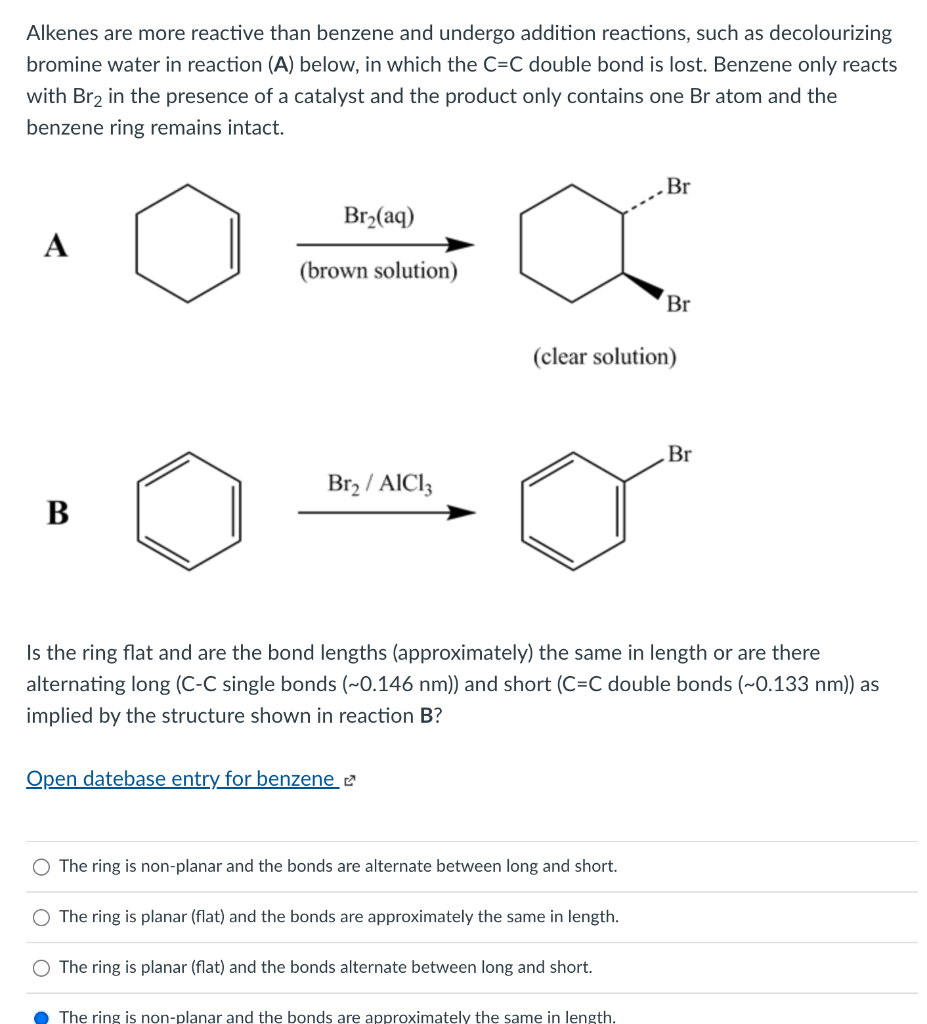
Alkenes are more reactive than benzene and undergo addition reactions, such as decolourizing bromine water in reaction (A) below, in which the C=C double bond is lost. Benzene only reacts with Br2 in the presence of a catalyst and the product only contains one Br atom and the benzene ring remains intact. Br Br2(aq) A ? (brown solution) Br (clear solution) Br Br2 / AICI: B Is the ring flat and are the bond lengths (approximately) the same in length or are there alternating long (C-C single bonds (~0.146 nm)) and short (C=C double bonds (~0.133 nm)) as implied by the structure shown in reaction B? Open datebase entry for benzene 2 The ring is non-planar and the bonds are alternate between long and short. The ring is planar (flat) and the bonds are approximately the same in length. The ring is planar (flat) and the bonds alternate between long and short. The ring is non-planar and the bonds are approximately the same in length.
Expert Answer
Benzene ring is planar and all bond lengths are equal , as there is delocali