Home /
Expert Answers /
Chemistry /
acids-bases-a-write-the-ka-expression-for-each-of-the-following-acids-which-is-strongest-how-pa382
(Solved): Acids \& Bases: a. Write the Ka expression for each of the following acids. Which is strongest? How ...
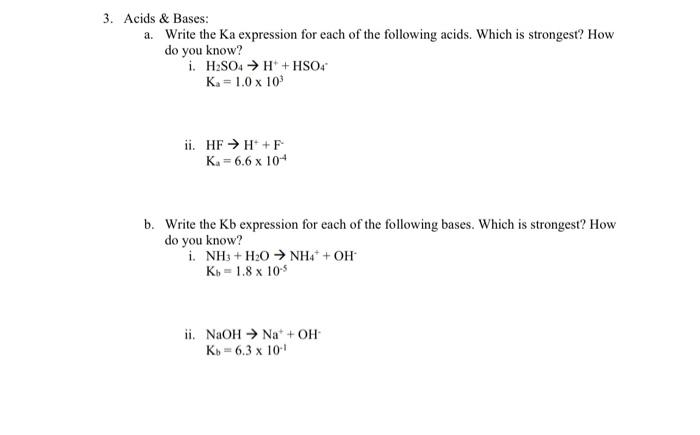
Acids \& Bases: a. Write the Ka expression for each of the following acids. Which is strongest? How do you know? i. \( \mathrm{H}_{2} \mathrm{SO}_{4} \rightarrow \mathrm{H}^{+}+\mathrm{HSO}_{4}{ }^{*} \) \( \mathrm{K}_{\mathrm{a}}=1.0 \times 10^{3} \) ii. \( \mathrm{HF} \rightarrow \mathrm{H}^{+}+\mathrm{F}^{-} \) \( K_{a}=6.6 \times 10^{-4} \) b. Write the Kb expression for each of the following bases. Which is strongest? How do you know? i. \( \mathrm{NH}_{3}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{NH}_{4}{ }^{+}+\mathrm{OH}^{-} \) \( \mathrm{K}_{b}=1.8 \times 10^{-5} \) ii. \( \mathrm{NaOH} \rightarrow \mathrm{Na}^{+}+\mathrm{OH}^{-} \) \( K_{b}=6.3 \times 10^{-1} \)