Home /
Expert Answers /
Chemistry /
acid-catalyzed-hydration-of-the-carbon-carbon-double-bond-in-ketene-i-h2c-c-o-gives-acetic-acid-pa115
(Solved): Acid-catalyzed hydration of the carbon-carbon double bond in ketene I(H2C=C=O) gives acetic acid ...
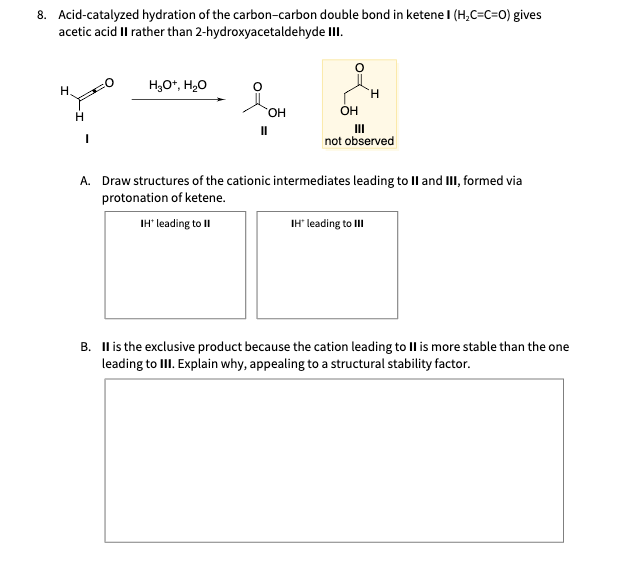
Acid-catalyzed hydration of the carbon-carbon double bond in ketene gives acetic acid II rather than 2-hydroxyacetaldehyde III. II III not observed A. Draw structures of the cationic intermediates leading to II and III, formed via protonation of ketene. B. II is the exclusive product because the cation leading to II is more stable than the one leading to III. Explain why, appealing to a structural stability factor.
Expert Answer
The acid-catalyzed hydration of a carbon-carbon double bond in ketene primarily results in the forma...