Home /
Expert Answers /
Chemistry /
acid-catalyzed-addition-of-water-to-an-alkene-yields-an-aicohol-with-markovnikoy-regiochernistry-t-pa446
(Solved): Acid-catalyzed addition of water to an alkene yields an aicohol with Markovnikoy regiochernistry. T ...
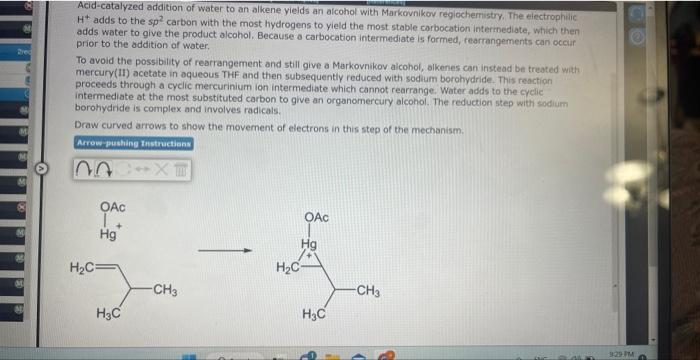
Acid-catalyzed addition of water to an alkene yields an aicohol with Markovnikoy regiochernistry. The electrophilic: \( \mathrm{H}^{+} \)adds to the \( s p^{2} \). carbon with the most hydrogens to yield the most stable carbocation intermediate, which then adds water to give the product alcohol, Because a carbocation intermediate is formed, rearrangements can occur. prior to the addition of water. To avoid the possibility of rearrangement and still give a Markovnikov alcohol, alkenes can instead be treated with mercury(II) acetate in aqueous. THF and then subsequently reduced with sodium borahydride. This reaction. proceeds through a cyclic mercurinium ion intermediate which cannot rearrange. Water adds to the cyclic intermediate at the most substituted carbon to give an organomercury alcohol. The reduction step with sadium. borohydride is complex and involves radicals. Draw curved arrows to show the movernent of electrons in this step of the mechanism.