Home /
Expert Answers /
Chemistry /
acetylene-c2h2-can-be-converted-to-ethane-c2h6-by-a-process-known-as-hydrogenation-pa544
(Solved): Acetylene, C2H2, can be converted to ethane, C2H6, by a process known as hydrogenation ...
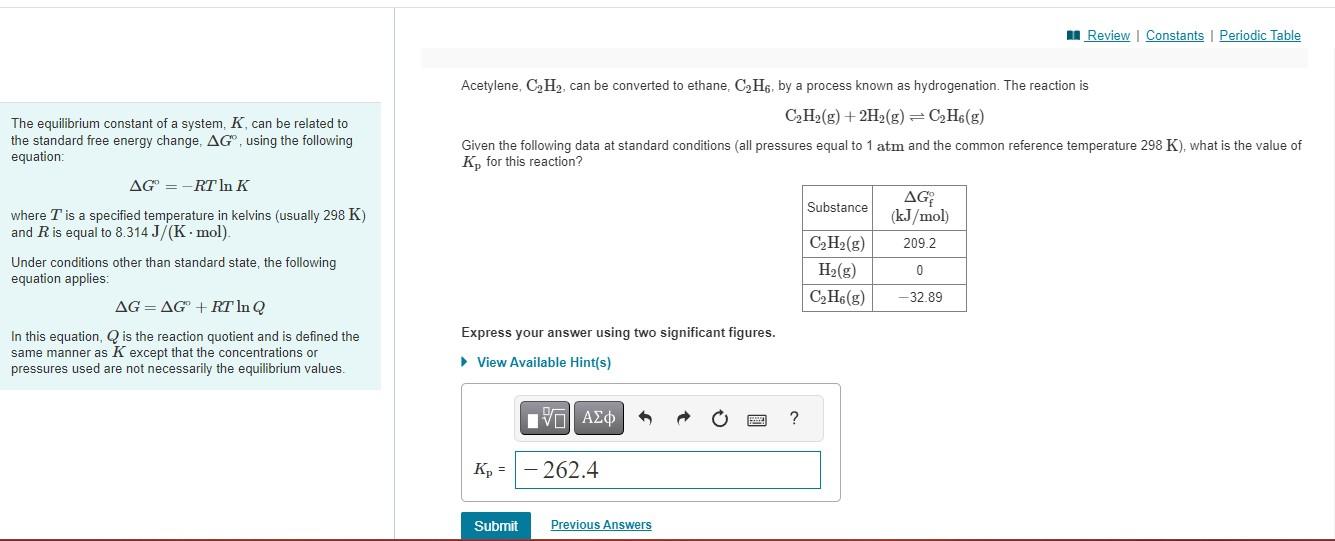
Acetylene, , can be converted to ethane, , by a process known as hydrogenation. The reaction is The equilibrium constant of a system, , can be related to the standard free energy change, , using the following Given the following data at standard conditions (all pressures equal to and the common reference temperature ), what is the value of equation: for this reaction? where is a specified temperature in kelvins (usually ) and is equal to . Under conditions other than standard state, the following equation applies: In this equation, is the reaction quotient and is defined the Express your answer using two significant figures. same manner as except that the concentrations or pressures used are not necessarily the equilibrium values.
Expert Answer
We know that, ?G0= -RTlnK ..........(1)Here the free energies of all reactants and products are given.The total free energhttps://media.cheggcdn.com/media/489/48907419-0654-48d7-9b95-4ce60b816b57/phplANfjJy of the reactants = 1*209.2+4*0 = 209.2 kJThe total free energy of the products = 1*(-32.89) = -32.89 kJ