Home /
Expert Answers /
Chemistry /
a-the-cis-and-trans-isomers-of-1-bromo-4-tert-butylcyclohexane-react-at-different-rates-with-pa971
(Solved): a. The cis and trans isomers of 1-bromo-4-tert-butylcyclohexane react at different rates with \( \ ...
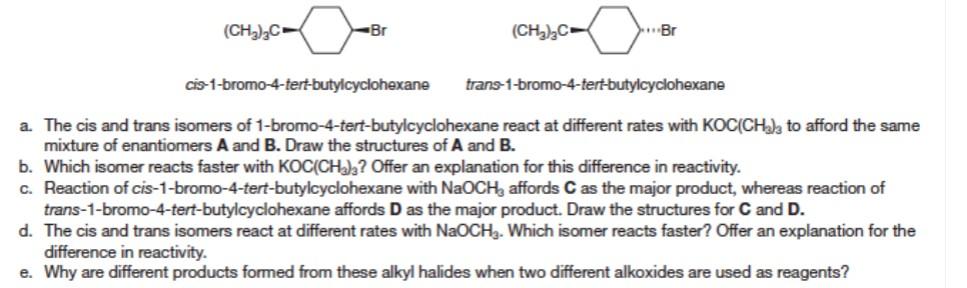
a. The cis and trans isomers of 1-bromo-4-tert-butylcyclohexane react at different rates with \( \mathrm{KOC}\left(\mathrm{CH}_{3}\right)_{3} \) to afford the same mixture of enantiomers \( \mathbf{A} \) and \( \mathbf{B} \). Draw the structures of \( \mathbf{A} \) and \( \mathbf{B} \). b. Which isomer reacts faster with \( \mathrm{KOC}\left(\mathrm{CH}_{3}\right)_{3} \) ? Offer an explanation for this difference in reactivity. c. Reaction of cis-1-bromo-4-tert-butylcyclohexane with \( \mathrm{NaOCH}_{3} \) affords \( \mathbf{C} \) as the major product, whereas reaction of trans-1-bromo-4-tert-butylcyclohexane affords \( \mathbf{D} \) as the major product. Draw the structures for \( \mathbf{C} \) and \( \mathbf{D} \). d. The cis and trans isomers react at different rates with \( \mathrm{NaOCH}_{3} \). Which isomer reacts faster? Offer an explanation for the difference in reactivity. e. Why are different products formed from these alkyl halides when two different alkoxides are used as reagents?