Home /
Expert Answers /
Chemistry /
a-solution-of-benzoic-acid-was-titrated-with-a-solution-of-sodium-hydroxide-naoh-and-the-ph-was-me-pa962
(Solved): A solution of benzoic acid was titrated with a solution of sodium hydroxide (NaOH) and the pH was me ...
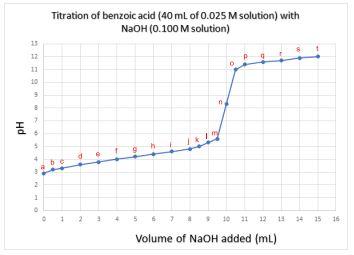
A solution of benzoic acid was titrated with a solution of sodium hydroxide (NaOH) and the pH was measured at a series of points according to the plot below.
a. Give the letter corresponding to the equivalence point.
b. Estimate the pH at the equivalence point.
c. Give the letter(s) corresponding to the buffer region.
d. Estimate the pKa of benzoic acid under the conditions of this titration and briefly describe how you determined this.
e. Based on the table of indicators shown below, which indicator would you pick to determine the equivalence point of the benzoic acid – NaOH titration? Explain your answer.
Titration of benzoic acid ( \( 40 \mathrm{~mL} \) of \( 0.025 \mathrm{M} \) solution) with \( \mathrm{NaOH} \) (0.100 M solution)