Home /
Expert Answers /
Physics /
a-sample-of-radium-88-224-ra-atomic-mass-224-020186u-t-1-2-3-66-days-contains-n-0-1-0-pa480
(Solved): A sample of radium _(88)^(224)Ra (atomic mass =224.020186u,T_((1)/(2))=3.66 days) contains N_(0)=1.0 ...
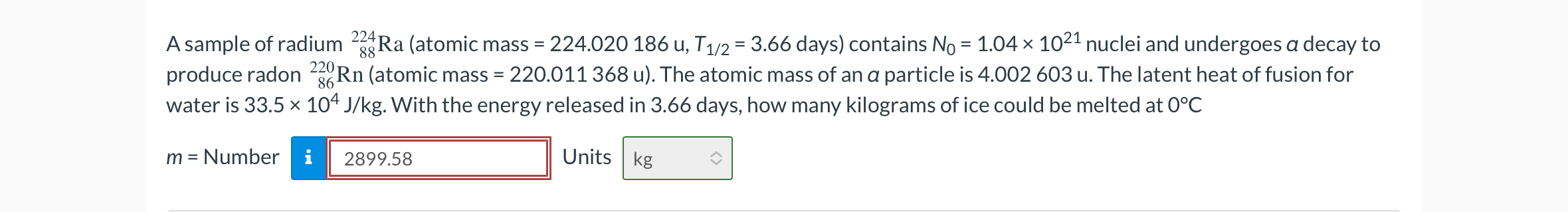
A sample of radium
_(88)^(224)Ra(atomic mass
=224.020186u,T_((1)/(2))=3.66days) contains
N_(0)=1.04\times 10^(21)nuclei and undergoes
adecay to produce radon
_(86)^(220)Rn(atomic mass
=220.011368u). The atomic mass of an
aparticle is 4.002603 u . The latent heat of fusion for water is
33.5\times 10^(4)(J)/(k)g. With the energy released in 3.66 days, how many kilograms of ice could be melted at
0\deg C
m=Number Units