Home /
Expert Answers /
Chemistry /
a-sample-of-iron-which-has-a-specific-heat-capacity-of-0-449-mathrm-j-cdot-mathrm-g-1-pa510
(Solved): A sample of iron, which has a specific heat capacity of \( 0.449 \mathrm{~J} \cdot \mathrm{g}^{-1} ...
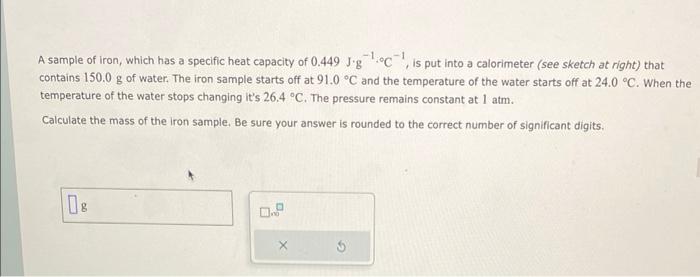
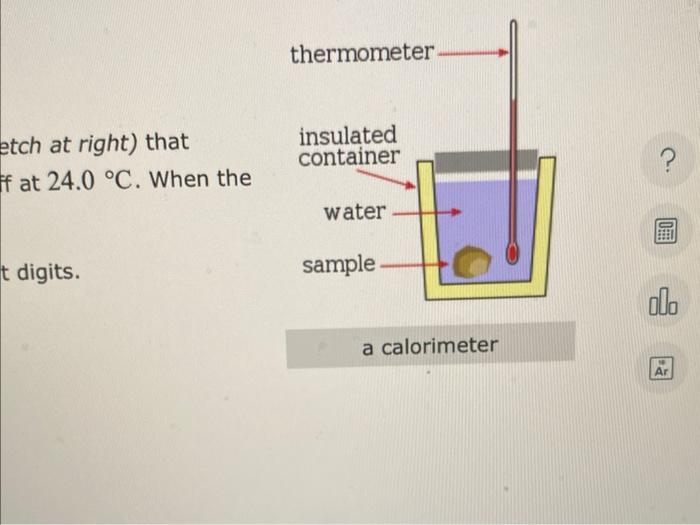
A sample of iron, which has a specific heat capacity of \( 0.449 \mathrm{~J} \cdot \mathrm{g}^{-1} \cdot{ }^{\circ} \mathrm{C}-1 \), is put into a calorimeter (see sketch at right) that contains \( 150.0 \mathrm{~g} \) of water. The iron sample starts off at \( 91.0^{\circ} \mathrm{C} \) and the temperature of the water starts off at \( 24.0{ }^{\circ} \mathrm{C} \). When the temperature of the water stops changing it's \( 26.4^{\circ} \mathrm{C} \). The pressure remains constant at \( 1 \mathrm{~atm} \). Calculate the mass of the iron sample. Be sure your answer is rounded to the correct number of significant digits.
etch at right) that If at \( 24.0^{\circ} \mathrm{C} \). When the digits.