Home /
Expert Answers /
Chemistry /
a-monatomic-ion-with-a-charge-of-2-has-an-electronic-configuration-of-1-s-2-2-s-2-2-p-pa219
(Solved): A monatomic ion with a charge of \( +2 \) has an electronic configuration of \( 1 s^{2} 2 s^{2} 2 p ...
![\[
\text { F-N }
\]
The most polar bond is](https://media.cheggcdn.com/study/414/414b68d7-c3b0-4204-905e-ecaaf956aab2/image)
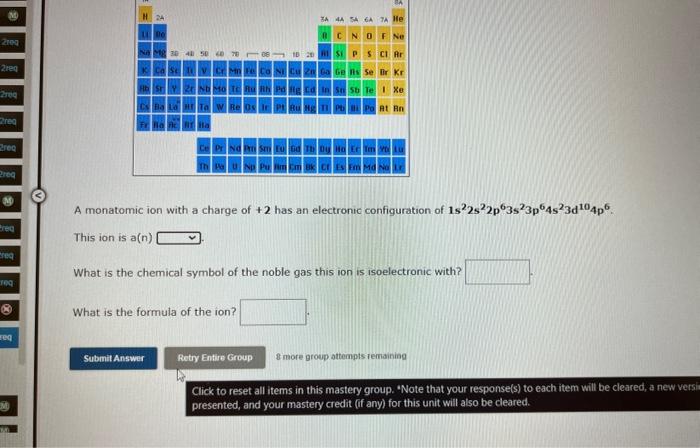
A monatomic ion with a charge of \( +2 \) has an electronic configuration of \( 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4 s^{2} 3 d^{10} 4 p^{6} \). This ion is \( a(n) \) What is the chemical symbol of the noble gas this ion is isoelectronic with? What is the formula of the ion? 8 more group attempts remaining Click to reset all items in this mastery group. "Note that your response(s) to each item will be cleared, a new vers presented, and your mastery credit (ff any) for this unit will also be cleared.
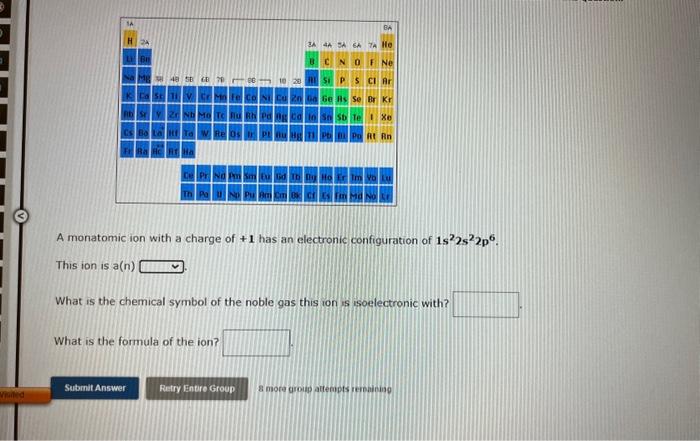
A monatomic ion with a charge of \( +1 \) has an electronic configuration of \( 1 s^{2} 2 s^{2} 2 p^{6} \). This ion is \( a(n) \) What is the chemical symbol of the noble gas this ion is isoelectronic with? What is the formula of the ion?
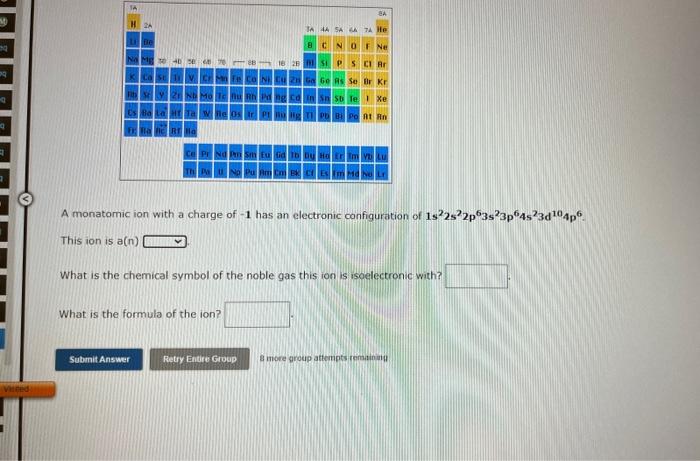
A monatomic ion with a charge of \( -1 \) has an electronic configuration of \( 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4 s^{2} 3 d^{10} 4 p^{6} \). This ion is a(n) What is the chemical symbol of the noble gas this ion is isoelectronic with? What is the formula of the ion?
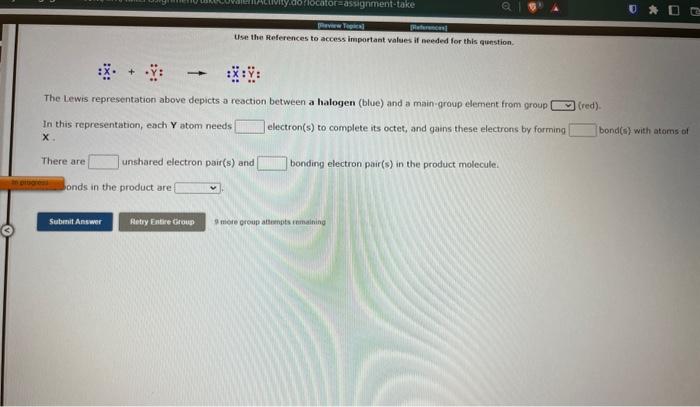
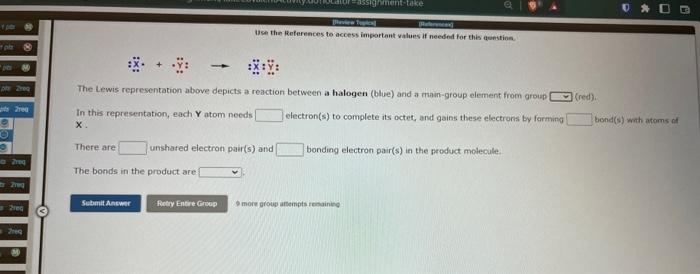
Use the Beferences to access inpartant values if needed for this aienstion. \[ : \ddot{x} \cdot+\ddot{\mathrm{x}}: \rightarrow i \ddot{\mathrm{x}}: \ddot{\mathrm{y}}: \] The Lewis representation above depicts a reaction between a halogen (blue) and a main-group element from groupl In this representation, each \( Y \) atom needs electron(s) to complete its octet, and gains these electrons by forming bond(s) with atoms of There are unshared electron pair(s) and bonding electron pair(s) in the product molecule. onds in the product are 9 ticie group atsimpts teimaining
Hse than References to access important values if neednd for this quenstina. The Lewis representation above depicts a reaction between a halogen (blue) and a main-group element from aroup In this representation, each \( \mathbf{Y} \) atom needs electron(s) to complete its octet, and gains these electrons by farming bond(s) with atoms of There are unshared electron pair(s) and bonding electron pair(s) in the product molecule. The bonds in the product are
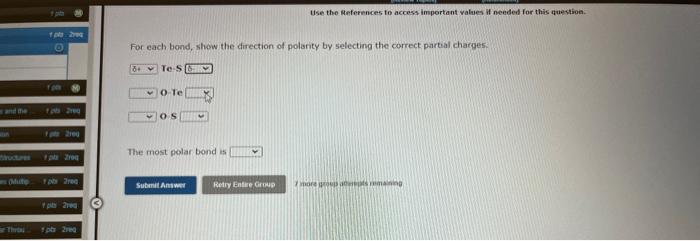
Te. S O.Te () 4 The most polar bond is
\[ \text { F-N } \] The most polar bond is
Expert Answer
As you have not specified which questions to answer so according to chegg honor code i am doing first and second and third question 1. electronic configuration given is 1s22s22p63s23p64s23d104p6 with a positive charge of +2 This ion is a