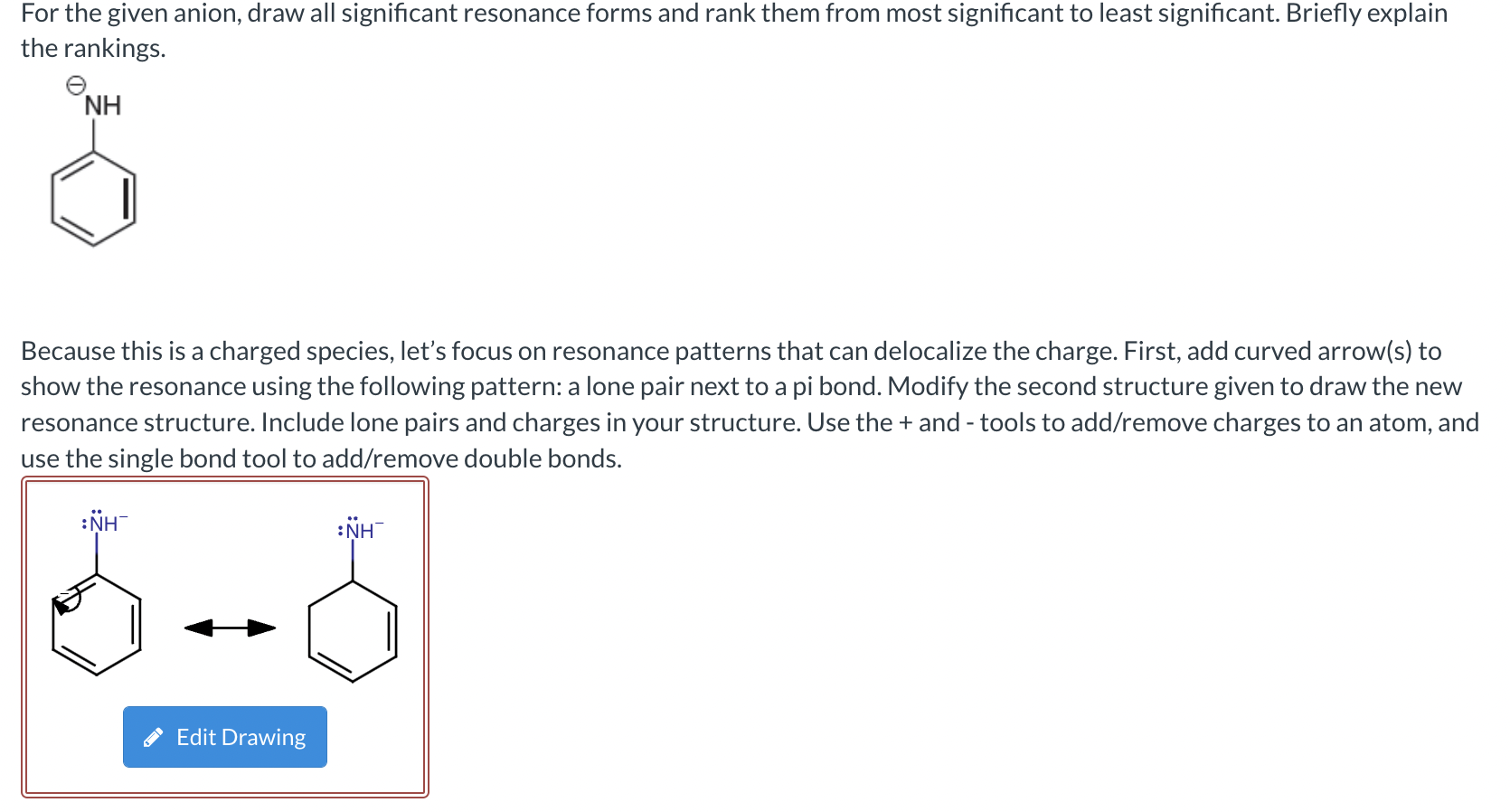
(Solved): a) For the given anion, draw all significant resonance forms and rank them from most significant to ...
a) For the given anion, draw all significant resonance forms and rank them from most significant to least significant. Briefly explain the rankings. Because this is a charged species, let’s focus on resonance patterns that can delocalize the charge. First, add curved arrow(s) to show the resonance using the following pattern: a lone pair next to a pi bond. Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.
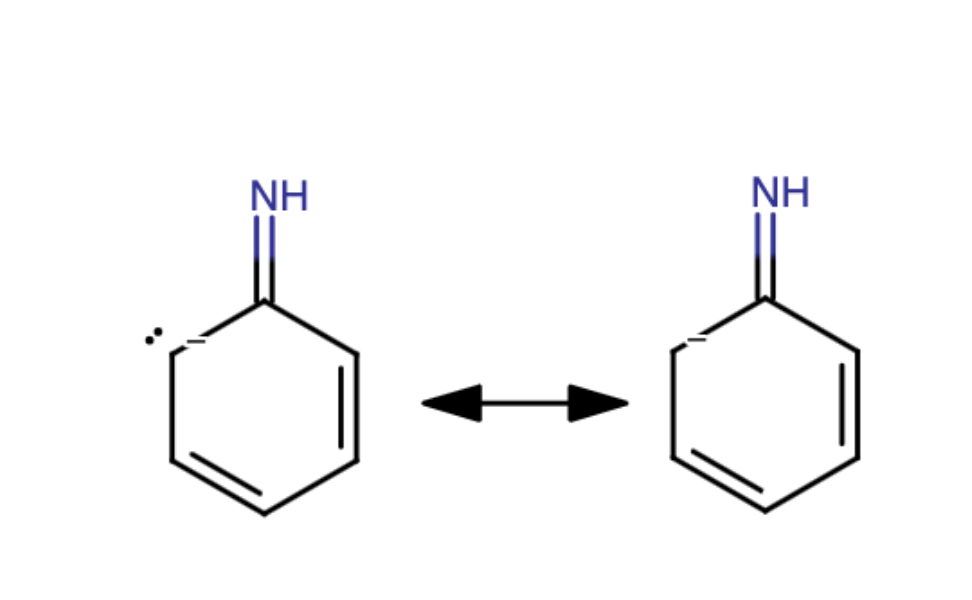
b) Next, add curved arrow(s) to show the resonance using the same pattern: a lone pair next to a pi bond. Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.