Home /
Expert Answers /
Chemistry /
a-chemist-dissolved-an-11-5-g-sample-of-koh-in-100-0-grams-of-water-in-a-coffee-cup-calorimet-pa259
(Solved): A chemist dissolved an 11.5-g sample of KOH in \( 100.0 \) grams of water in a coffee cup calorimet ...
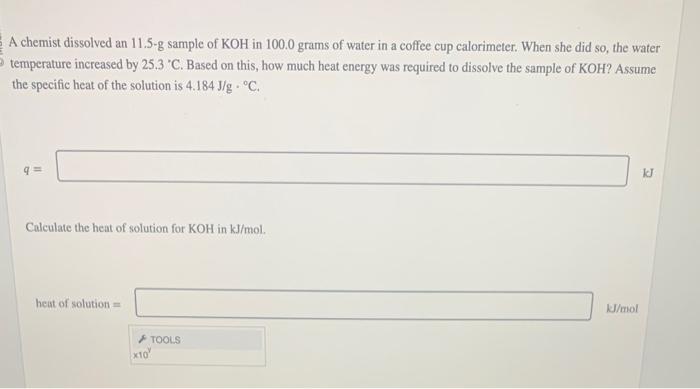
A chemist dissolved an 11.5-g sample of KOH in \( 100.0 \) grams of water in a coffee cup calorimeter. When she did so, the water temperature increased by \( 25.3^{\circ} \mathrm{C} \). Based on this, how much heat energy was required to dissolve the sample of KOH? Assume the specific heat of the solution is \( 4.184 \mathrm{~J} / \mathrm{g} \cdot{ }^{\circ} \mathrm{C} \). \( q \) Calculate the heat of solution for \( \mathrm{KOH} \) in \( \mathrm{kJ} / \mathrm{mol} \). heat of solution \( = \) \( \mathrm{kJ} / \mathrm{mol} \)