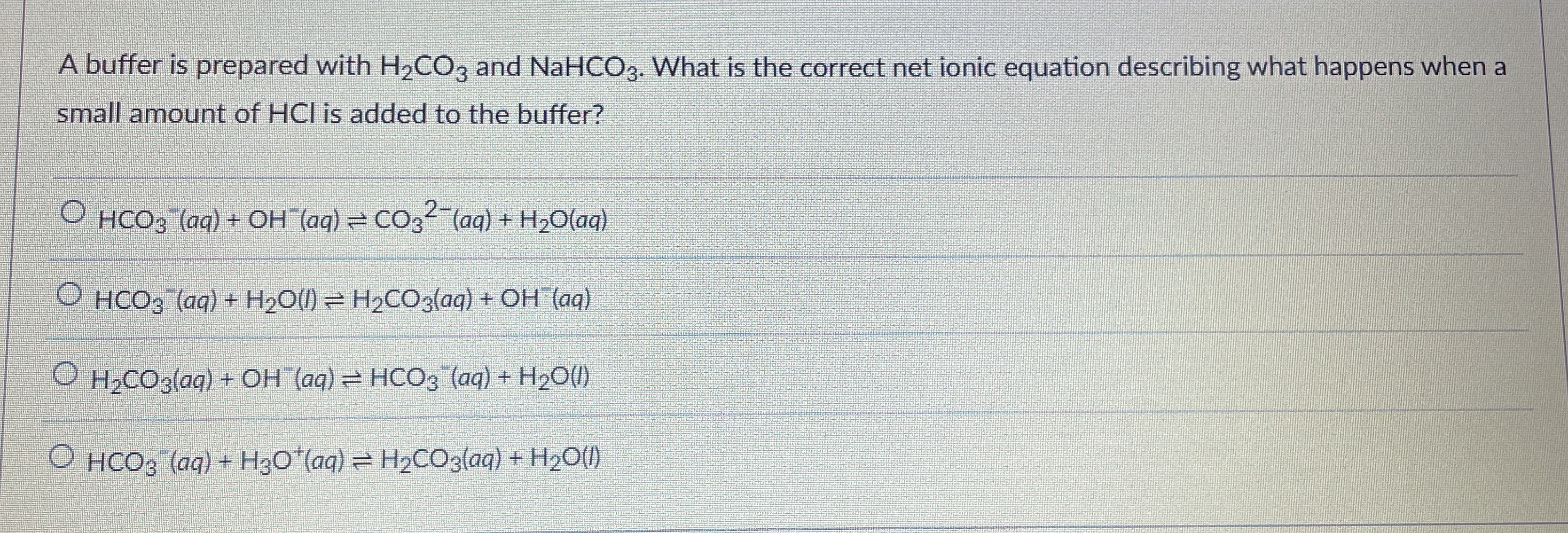Home /
Expert Answers /
Chemistry /
a-buffer-is-prepared-with-h-2-co-3-and-nahco-3-what-is-the-correct-net-ionic-equation-describi-pa680
(Solved): A buffer is prepared with H_(2)CO_(3) and NaHCO_(3). What is the correct net ionic equation describi ...
A buffer is prepared with
H_(2)CO_(3)and
NaHCO_(3). What is the correct net ionic equation describing what happens when a small amount of HCl is added to the buffer?
HCO_(3)^(-)(aq)+OH^(-)(aq)⇌CO_(3)^(2-)(aq)+H_(2)O(aq)
HCO_(3)^(-)(aq)+H_(2)O(l)⇌H_(2)CO_(3)(aq)+OH^(-)(aq)
H_(2)CO_(3)(aq)+OH^(-)(aq)⇌HCO_(3)^(-)(aq)+H_(2)O(I)l