Home /
Expert Answers /
Chemistry /
a-body-centered-cubic-unit-cell-has-a-volume-of-1-781023cm3-find-the-radius-of-the-atom-in-pa950
(Solved): A body-centered cubic unit cell has a volume of 1.781023cm3. Find the radius of the atom in ...
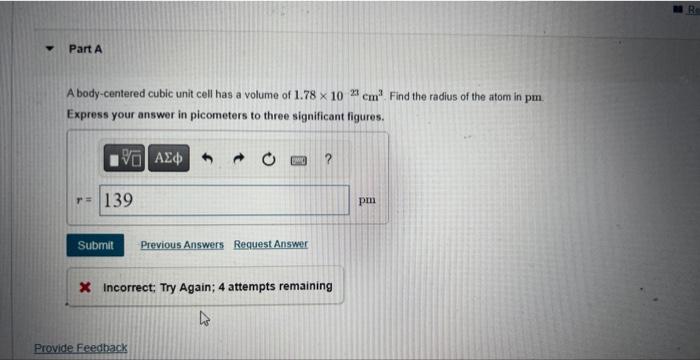
A body-centered cubic unit cell has a volume of . Find the radius of the atom in . Express your answer in picometers to three significant figures. 3. Incorrect; Try Again; 4 attempts remaining
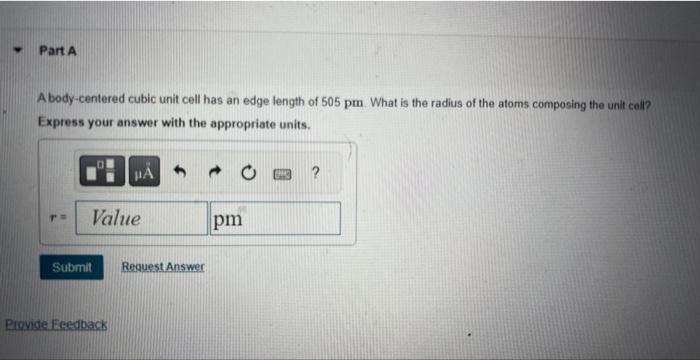
A body-centered cubic unit cell has an edge length of . What is the radius of the atoms composing the unit cell? Express your answer with the appropriate units.