Home /
Expert Answers /
Chemistry /
a-b-thank-you-use-standard-reduction-potentials-to-calculate-the-standard-free-energy-ch-pa906
(Solved): A) B) Thank you!!! Use standard reduction potentials to calculate the standard free energy ch ...
A)
B)
Thank you!!!
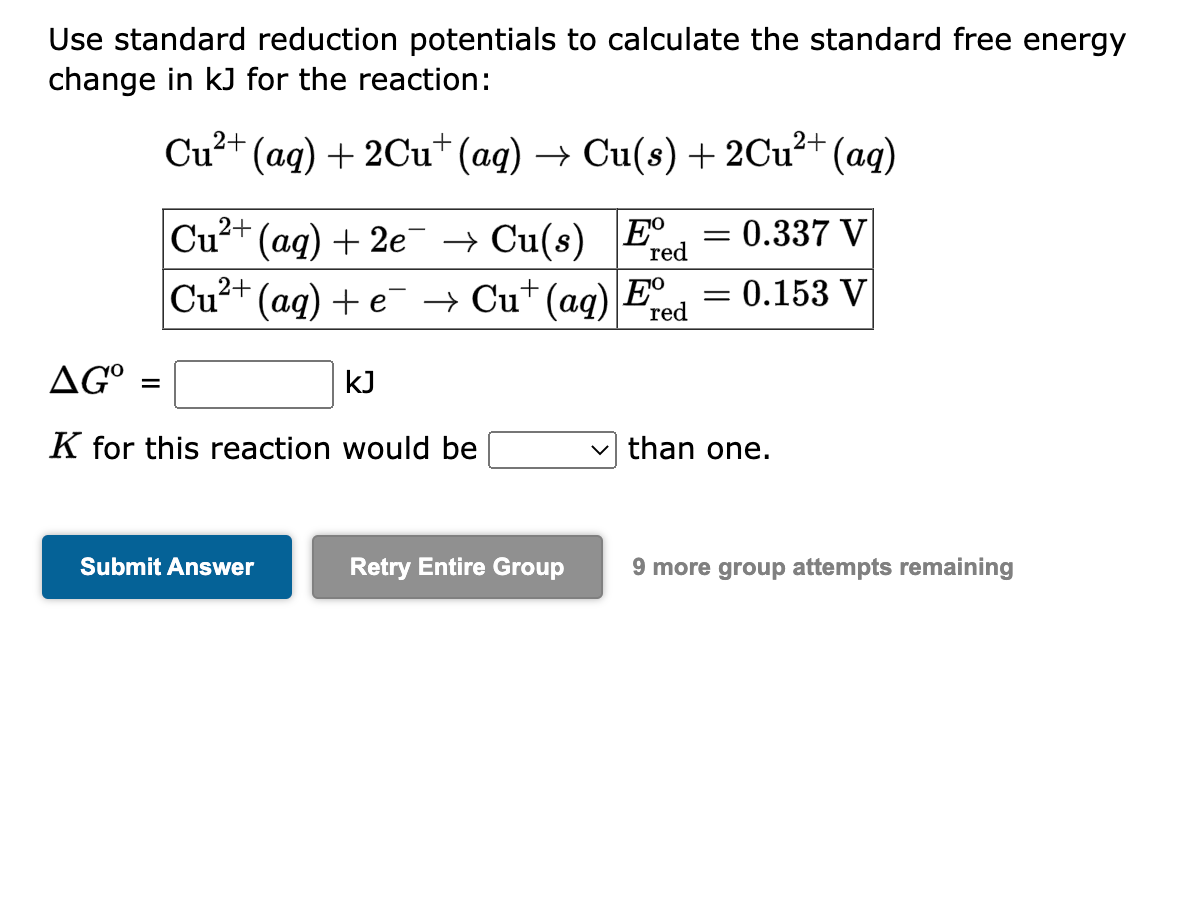
Use standard reduction potentials to calculate the standard free energy change in \( \mathrm{kJ} \) for the reaction: \[ \mathrm{Cu}^{2+}(a q)+2 \mathrm{Cu}^{+}(a q) \rightarrow \mathrm{Cu}(s)+2 \mathrm{Cu}^{2+}(a q) \] \[ \Delta G^{\mathrm{o}}=\quad \mathrm{kJ} \] \( K \) for this reaction would be than one.
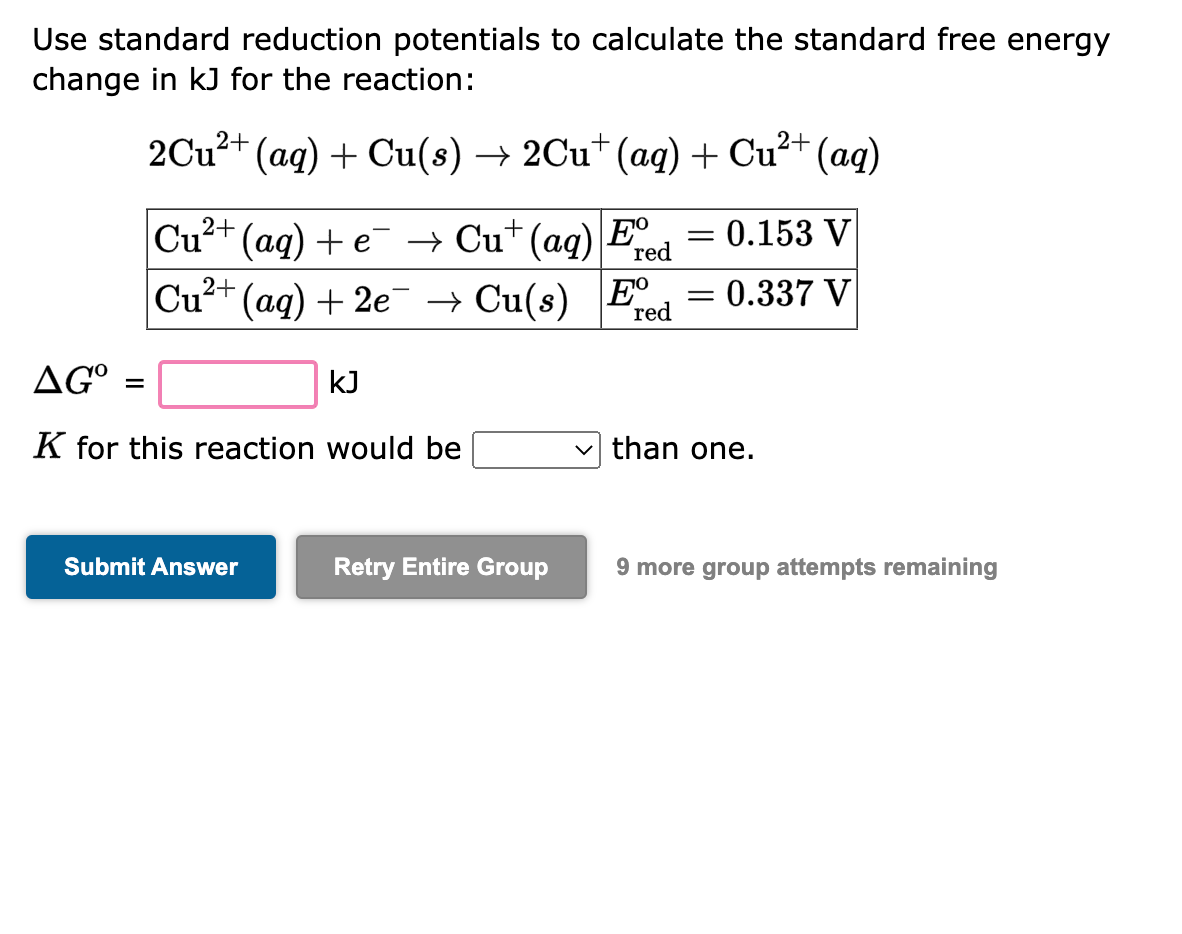
Use standard reduction potentials to calculate the standard free energy change in \( \mathrm{kJ} \) for the reaction: \[ \begin{array}{l} 2 \mathrm{Cu}^{2+}(a q)+\mathrm{Cu}(s) \rightarrow 2 \mathrm{Cu}^{+}(a q)+\mathrm{Cu}^{2+}(a q) \\ \begin{array}{|l|l|} \hline \mathrm{Cu}^{2+}(a q)+e^{-} \rightarrow \mathrm{Cu}^{+}(a q) & E_{\text {red }}^{\circ}=0.153 \mathrm{~V} \\ \hline \mathrm{Cu}^{2+}(a q)+2 e^{-} \rightarrow \mathrm{Cu}(s) & E_{\text {red }}^{\circ}=0.337 \mathrm{~V} \\ \hline \end{array} \\ \Delta G^{\mathrm{o}}=\quad \mathrm{kJ} \\ \end{array} \] \( K \) for this reaction would be than one. 9 more group attempts remaining