Home /
Expert Answers /
Chemistry /
7-the-9-text-th-hr-chemistry-class-produced-the-following-graph-when-they-were-measuri-pa456
(Solved): 7. The \( 9^{\text {th }} \) Hr chemistry class produced the following graph when they were measuri ...
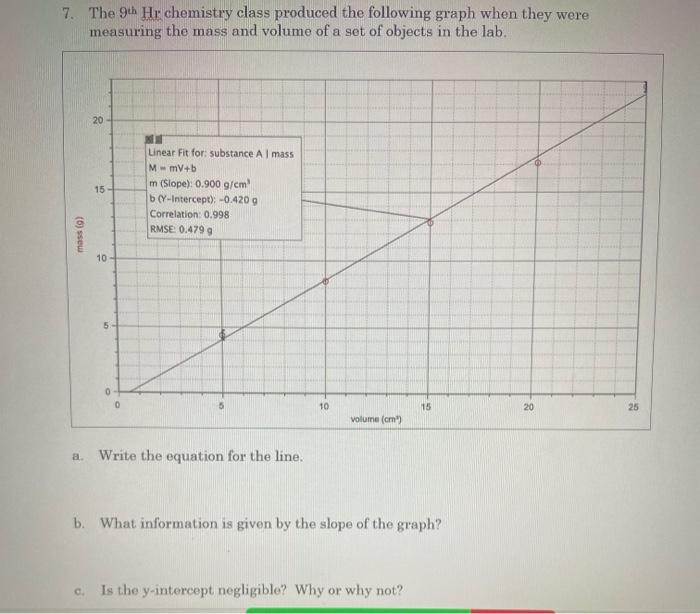

7. The \( 9^{\text {th }} \) Hr chemistry class produced the following graph when they were measuring the mass and volume of a set of objects in the lab. a. Write the equation for the line. b. What information is given by the slope of the graph? c. Is the y-intercept negligible? Why or why not?
d. What would you predict would happen if you were to put one of the objects in water? Explain. e. What would you expect to be the mass of a \( 45 \mathrm{~cm}^{3} \) piece of the same substance?
Expert Answer
1.Equation for linear line is : y = mx + b Here y is M i.e., mass and x is V ( volume) Therefore in this case equation will be m is the slope of the g