Home /
Expert Answers /
Chemistry /
7-a-key-step-in-the-biosynthesis-of-triglycerides-is-the-conversion-of-glycerol-to-glycerol-1-pho-pa931
(Solved): 7.) A key step in the biosynthesis of triglycerides is the conversion of glycerol to glycerol-1-pho ...
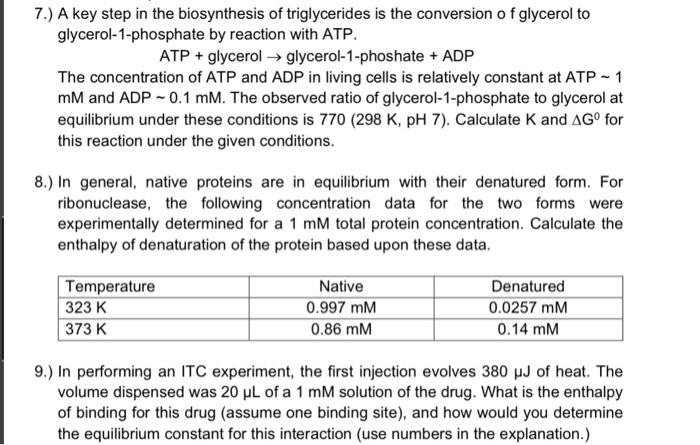
7.) A key step in the biosynthesis of triglycerides is the conversion of glycerol to glycerol-1-phosphate by reaction with ATP. ATP \( + \) glycerol \( \rightarrow \) glycerol-1-phoshate \( + \) ADP The concentration of ATP and ADP in living cells is relatively constant at ATP \( \sim 1 \) \( \mathrm{mM} \) and \( \mathrm{ADP} \sim 0.1 \mathrm{mM} \). The observed ratio of glycerol-1-phosphate to glycerol at equilibrium under these conditions is \( 770(298 \mathrm{~K}, \mathrm{pH} 7) \). Calculate \( \mathrm{K} \) and \( \Delta \mathrm{G}^{0} \) for this reaction under the given conditions. 8.) In general, native proteins are in equilibrium with their denatured form. For ribonuclease, the following concentration data for the two forms were experimentally determined for a \( 1 \mathrm{mM} \) total protein concentration. Calculate the enthalpy of denaturation of the protein based upon these data. 9.) In performing an ITC experiment, the first injection evolves \( 380 \mu \mathrm{J} \) of heat. The volume dispensed was \( 20 \mu \mathrm{L} \) of a \( 1 \mathrm{mM} \) solution of the drug. What is the enthalpy of binding for this drug (assume one binding site), and how would you determine the equilibrium constant for this interaction (use numbers in the explanation.)
Expert Answer
7) Glycerol + ATP ----------> Glycerol -1-phosphate + ADP K = [Glyph] [ADP] / [Gly] [ATP] but [Gly-ph]/[Gly] = 770 so: K = 770 x [ADP] / [ATP] K