Home /
Expert Answers /
Chemistry /
6-sodium-metal-reacts-with-chlorine-gas-to-produce-sodium-chloride-2-mathrm-na-mathrm-s-pa355
(Solved): 6. Sodium metal reacts with chlorine gas to produce sodium chloride. \[ 2 \mathrm{Na}_{(\mathrm{s}) ...
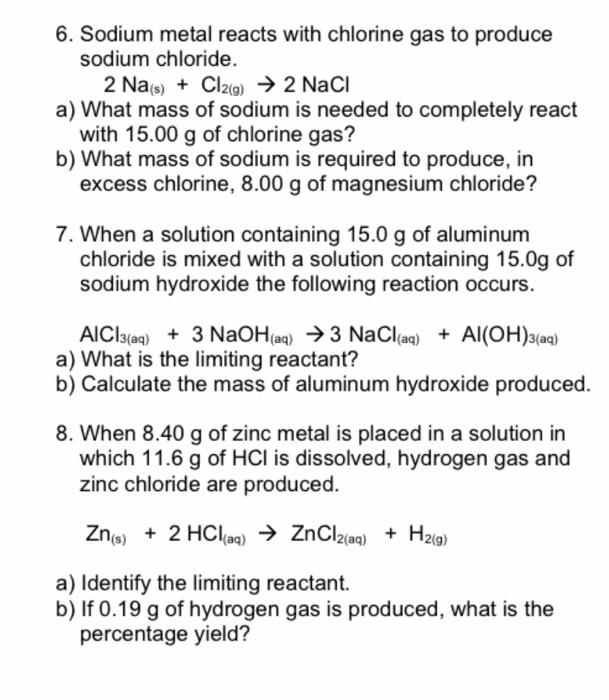
6. Sodium metal reacts with chlorine gas to produce sodium chloride. \[ 2 \mathrm{Na}_{(\mathrm{s})}+\mathrm{Cl}_{2(g)} \rightarrow 2 \mathrm{NaCl} \] a) What mass of sodium is needed to completely react with \( 15.00 \mathrm{~g} \) of chlorine gas? b) What mass of sodium is required to produce, in excess chlorine, \( 8.00 \mathrm{~g} \) of magnesium chloride? 7. When a solution containing \( 15.0 \mathrm{~g} \) of aluminum chloride is mixed with a solution containing \( 15.0 \mathrm{~g} \) of sodium hydroxide the following reaction occurs. \( \mathrm{AlCl}_{3(a \mathrm{a})}+3 \mathrm{NaOH}_{\text {(aq) }} \rightarrow 3 \mathrm{NaCl}_{\text {(aq) }}+\mathrm{Al}(\mathrm{OH})_{3(\mathrm{aq})} \) a) What is the limiting reactant? b) Calculate the mass of aluminum hydroxide produced. 8. When \( 8.40 \mathrm{~g} \) of zinc metal is placed in a solution in which \( 11.6 \mathrm{~g} \) of \( \mathrm{HCl} \) is dissolved, hydrogen gas and zinc chloride are produced. \[ \mathrm{Zn}_{\text {(s) }}+2 \mathrm{HCl}_{\text {(aq) }} \rightarrow \mathrm{ZnCl}_{2(\mathrm{aq})}+\mathrm{H}_{2(\mathrm{~g})} \] a) Identify the limiting reactant. b) If \( 0.19 \mathrm{~g} \) of hydrogen gas is produced, what is the percentage yield?