Home /
Expert Answers /
Chemical Engineering /
5-calculate-the-total-enthalpy-change-for-the-following-reaction-the-specific-heat-capacity-of-ic-pa180
(Solved): 5. Calculate the total enthalpy change for the following reaction. The specific heat capacity of ic ...
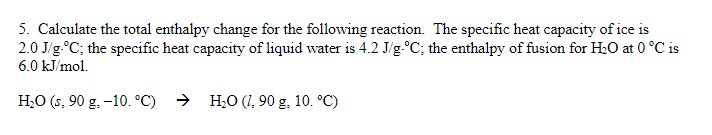
5. Calculate the total enthalpy change for the following reaction. The specific heat capacity of ice is ; the specific heat capacity of liquid water is ; the enthalpy of fusion for at is