Home /
Expert Answers /
Chemistry /
4-pb-s-pbso4-s-hg2so4-s-hg-l-a-write-the-cell-reaction-and-electrode-half-reactio-pa736
(Solved): 4. Pb(s)PbSO4(s)Hg2SO4 (s) Hg(l) (a) Write the cell reaction and electrode half-reactio ...
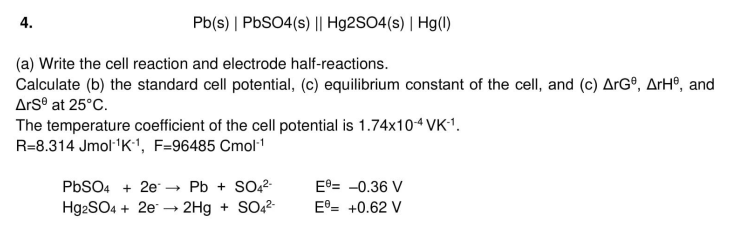
4. (s) (a) Write the cell reaction and electrode half-reactions. Calculate (b) the standard cell potential, (c) equilibrium constant of the cell, and (c) , and at . The temperature coefficient of the cell potential is .
Expert Answer
The given cell notation represents a galvanic cell...