Home /
Expert Answers /
Computer Science /
3-the-empirical-formula-of-sugar-is-ch2o-and-its-molar-mass-is-180-2g-mole-if-one-teaspoon-o-pa857
(Solved): 3) The empirical formula of sugar is CH2O and its molar mass is 180.2g/mole. If one teaspoon o ...
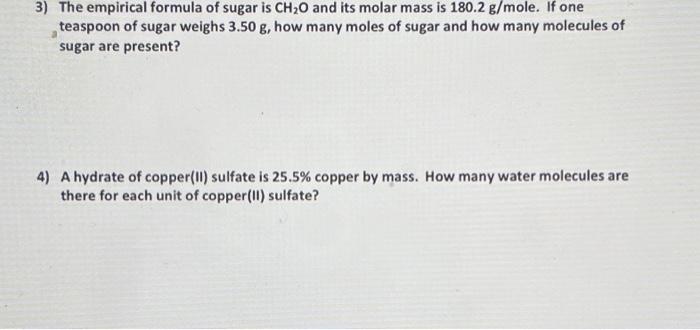
3) The empirical formula of sugar is and its molar mass is . If one teaspoon of sugar weighs , how many moles of sugar and how many molecules of sugar are present? 4) A hydrate of copper(II) sulfate is copper by mass. How many water molecules are there for each unit of copper(II) sulfate?