Home /
Expert Answers /
Mechanical Engineering /
3-60-as-shown-in-fig-p3-60-a-tank-open-to-the-atmosphere-initially-contains-2-lb-of-liquid-water-a-pa843
(Solved): 3.60 As shown in Fig. P3.60, a tank open to the atmosphere initially contains 2 lb of liquid water a ...
3.60 As shown in Fig. P3.60, a tank open to the atmosphere initially contains 2 lb of liquid water at 80°F and 0.4 lb of ice at 32°F. All of the ice melts as the tank contents attain equilibrium. If no significant heat transfer occurs between the tank contents and their surroundings, determine the final equilibrium temperature, in °F. For water, the spe- cific enthalpy change for a phase change from solid to liquid at 32°F and 1 atm is 144 Btu/lb. FIGURE P3.60 Patm 1 atm = Liquid water, m = 2 lb, initially at 80°F Ice, m = 0.4 lb (total mass), initially at 32°F
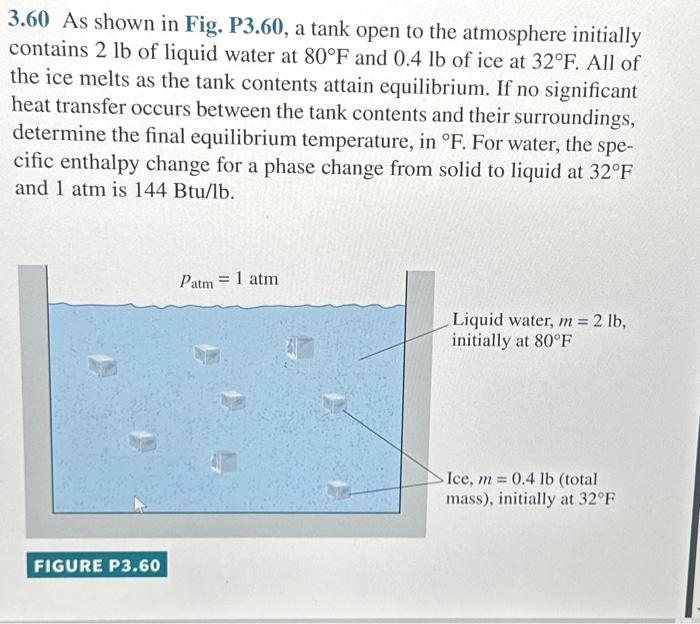
3.60 As shown in Fig. P3.60, a tank open to the atmosphere initially contains of liquid water at and of ice at . All of the ice melts as the tank contents attain equilibrium. If no significant heat transfer occurs between the tank contents and their surroundings, determine the final equilibrium temperature, in . For water, the specific enthalpy change for a phase change from solid to liquid at and is .