Home /
Expert Answers /
Chemistry /
2so32-sisio32-s2o32-in-the-above-redox-reaction-use-oxidation-numbers-to-i-pa521
(Solved): 2SO32+SiSiO32+S2O32 In the above redox reaction, use oxidation numbers to i ...
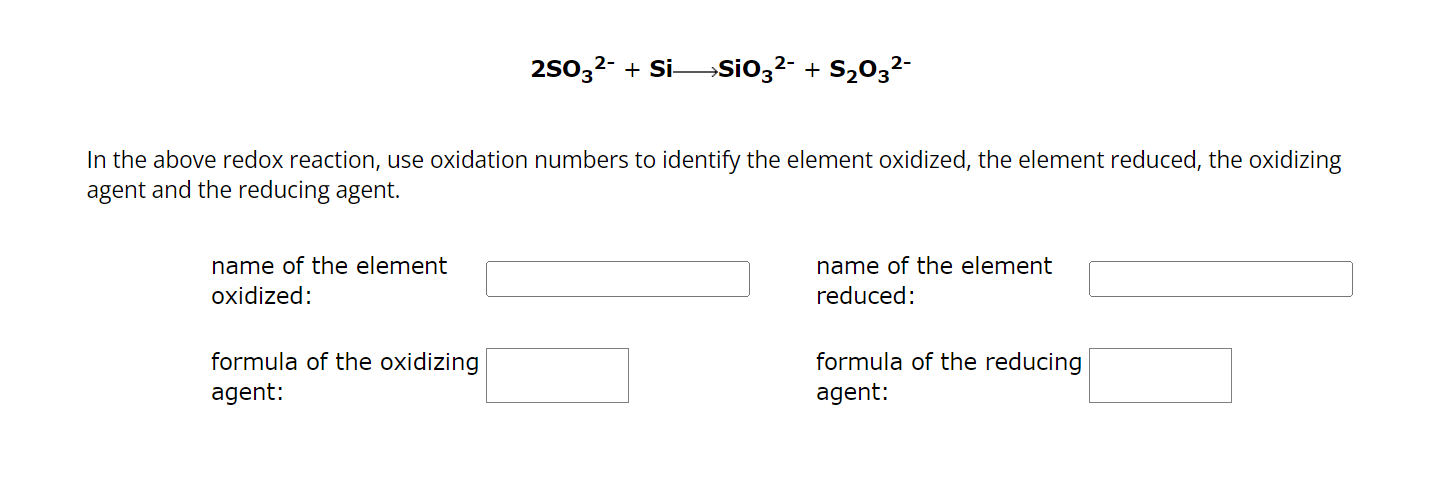
In the above redox reaction, use oxidation numbers to identify the element oxidized, the element reduced, the oxidizing agent and the reducing agent. name of the element oxidized: formula of the oxidizing agent: name of the element reduced: formula of the reducing agent: