Home /
Expert Answers /
Chemistry /
28-calculate-the-heats-of-combustion-for-the-following-reactions-from-the-st-pa339
(Solved): 28) Calculate the heats of combustion for the following reactions from the st ...
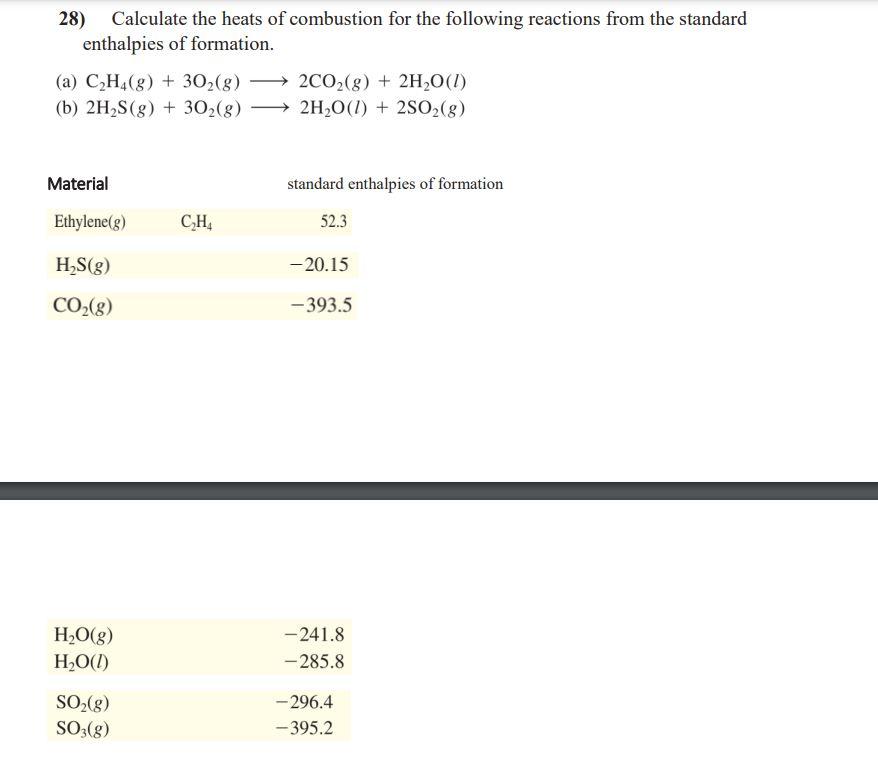
28) Calculate the heats of combustion for the following reactions from the standard enthalpies of formation. (a) \( \mathrm{C}_{2} \mathrm{H}_{4}(g)+3 \mathrm{O}_{2}(g) \longrightarrow 2 \mathrm{CO}_{2}(g)+2 \mathrm{H}_{2} \mathrm{O}(l) \) (b) \( 2 \mathrm{H}_{2} \mathrm{~S}(\mathrm{~g})+3 \mathrm{O}_{2}(g) \longrightarrow 2 \mathrm{H}_{2} \mathrm{O}(\mathrm{l})+2 \mathrm{SO}_{2}(g) \) Material standard enthalpies of formation