Home /
Expert Answers /
Chemistry /
2-on-diagram-shown-below-draw-one-hydrogen-bond-that-would-exist-between-molecule-1-and-2-then-pa746
(Solved): 2. On diagram shown below, draw one hydrogen bond that would exist between molecule 1 and 2 , then ...
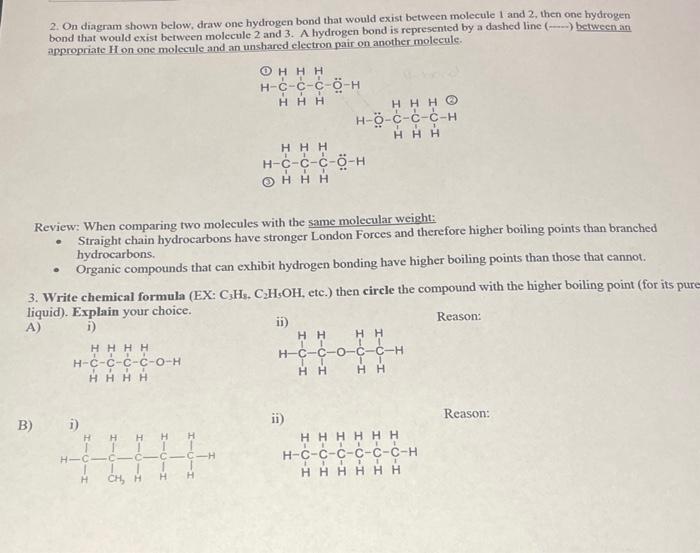
2. On diagram shown below, draw one hydrogen bond that would exist between molecule 1 and 2 , then one hydrogen bond that would exist between molecule 2 and 3 . A hydrogen bond is represented by a dashed line \( (-\ldots) \) between an appropriate H on one molecule and an unshared electron pair on another molecule. Review: When comparing two molecules with the same molecular weight: - Straight chain hydrocarbons have stronger London Forces and therefore higher boiling points than branched hydrocarbons. - Organic compounds that can exhibit hydrogen bonding have higher boiling points than those that cannot. 3. Write chemical formula (EX: \( \mathrm{C}_{3} \mathrm{H}_{5} \). \( \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH} \), etc.) then circle the compound with the higher boiling point (for its pi liquid). Explain your choice. A) i) Reason: B) ii Reason:
Expert Answer
Solution - a hydrogen bond is a primarily electrostatic force of attraction between a hydrogen atom which is covalently bound to a more electro-negati