Home /
Expert Answers /
Chemistry /
2-of-the-remaining-4-carbon-chain-to-make-2-methylbutane-this-is-a-structural-isomer-of-p-ac-pa974
(Solved): 2 of the remaining 4 -carbon chain to make 2 -methylbutane. This is a structural isomer of p; ac - ...
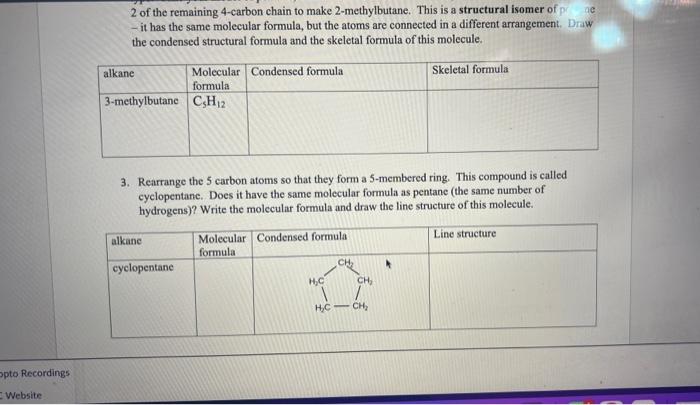
2 of the remaining 4 -carbon chain to make 2 -methylbutane. This is a structural isomer of p; ac - it has the same molecular formula, but the atoms are connected in a different arrangement. Draw the condensed structural formula and the skeletal formula of this molecule. 3. Rearrange the 5 carbon atoms so that they form a 5-membered ring. This compound is called cyclopentanc. Does it have the same molecular formula as pentane (the same number of hydrogens)? Write the molecular formula and draw the line structure of this molecule. spto Recordings Website
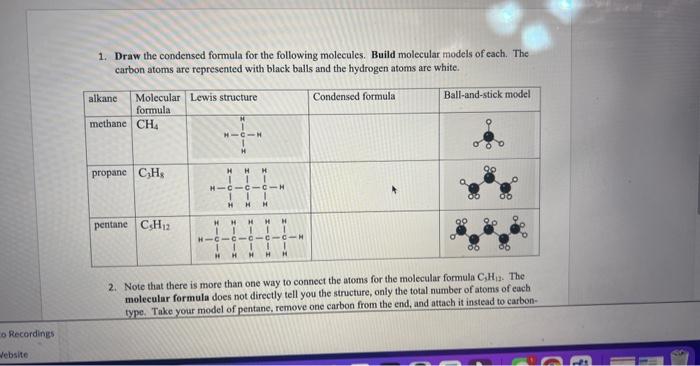
Draw the condensed formula for the following molecules. Build molecular models of each. The carbon atoms are represented with black balls and the hydrogen atoms are white. 2. Note that there is more than one way to connect the atoms for the molecular formula \( \mathrm{C}_{3} \mathrm{H}_{12} \). The molecular formula does not directly tell you the structure, only the total number of atoms of each type. Take your model of pentane, remove one carbon from the end, and attach it instead to carbon-