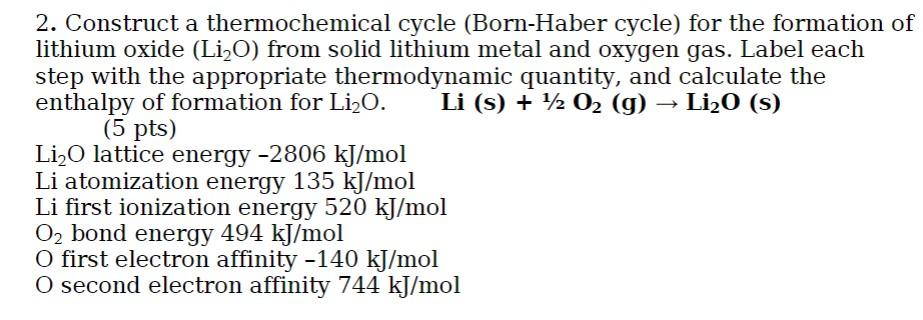Home /
Expert Answers /
Chemistry /
2-construct-a-thermochemical-cycle-born-haber-cycle-for-the-formation-of-lithium-oxide-li2o-f-pa733
(Solved): 2. Construct a thermochemical cycle (Born-Haber cycle) for the formation of lithium oxide (Li2O) f ...
2. Construct a thermochemical cycle (Born-Haber cycle) for the formation of lithium oxide (Li2O) from solid lithium metal and oxygen gas. Label each step with the appropriate thermodynamic quantity, and calculate the enthalpy of formation for Li20. Li (s) + 42 02 (g) ? Li20 (S) (5 pts) Li2O lattice energy -2806 kJ/mol Li atomization energy 135 kJ/mol Li first ionization energy 520 kJ/mol O2 bond energy 494 kJ/mol O first electron affinity -140 kJ/mol O second electron affinity 744 kJ/mol