Home /
Expert Answers /
Chemistry /
1r-3r-1-3-dichlorocyclohexane-a-c-cannot-flip-d-1r-3s-1-3-dichlorocyclohexane-a-cannot-flip-pa304
Expert Answer
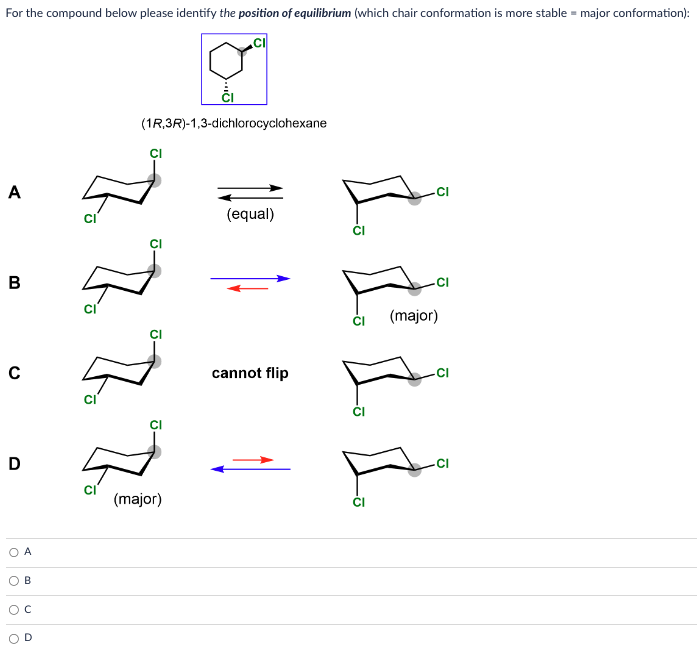
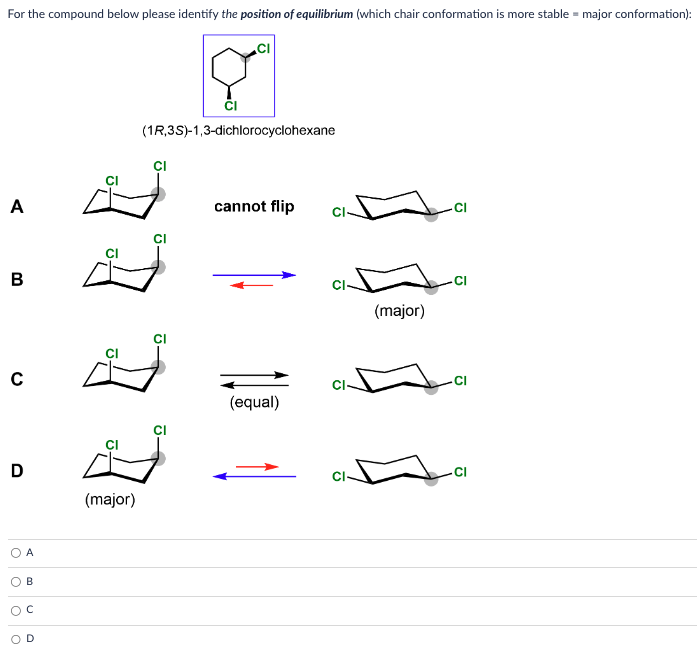
solution:The chair conformation is the most stable conformation that cyclohexane can adopt, there is enough thermal energy for it to also pass through less favorable conformations before returning to a different chair conformation. The passage of cyclohexane from one chair conformation to another, during which the axial substituents switch places with the equatorial substituents, is called a ring flip.Determining the more stable chair conformation becomes more complex when there are two or more substituents attached to the cyclohexane ring. To determine the stable chair conformation, the steric effects of each substituent, along with any additional steric interactions, must be taken into account for both chair conformations.In this section, the effect of conformations on the relative stability of disubstituted cyclohexanes is examined using the two principles:Substituents prefer equatorial rather than axial positions in order to minimize the steric strain created of 1,3-diaxial interactions.The more stable conformation will place the larger substituent in the equatorial position.