Home /
Expert Answers /
Physics /
1kg-of-ice-at-20c-is-taken-out-of-the-freezer-and-placed-in-a-pot-o-pa937
(Solved): 1kg of ice, at 20C, is taken out of the freezer and placed in a pot o ...
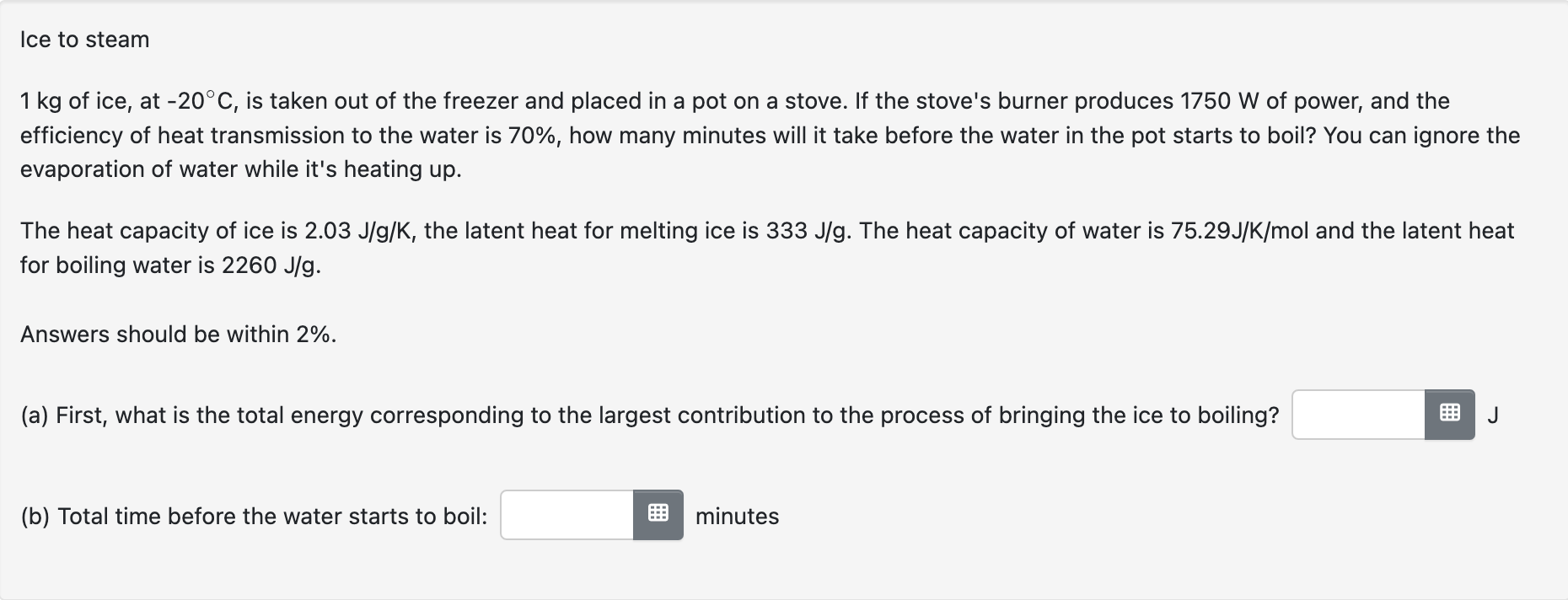
of ice, at , is taken out of the freezer and placed in a pot on a stove. If the stove's burner produces of power, and the efficiency of heat transmission to the water is , how many minutes will it take before the water in the pot starts to boil? You can ignore the evaporation of water while it's heating up. The heat capacity of ice is , the latent heat for melting ice is . The heat capacity of water is and the latent heat for boiling water is . Answers should be within . (a) First, what is the total energy corresponding to the largest contribution to the process of bringing the ice to boiling? (b) Total time before the water starts to boil: minutes
Expert Answer
Sol:(a) First, we need to find the total energy required to bring the ice to boiling. This energy consists of three components:The energy required to