Home /
Expert Answers /
Chemistry /
14-the-lewis-structure-of-chlorambucil-a-common-chemotherapy-drug-used-for-leukemia-and-various-ly-pa135
(Solved): 14.The Lewis structure of Chlorambucil, a common chemotherapy drug used for leukemia and various ly ...
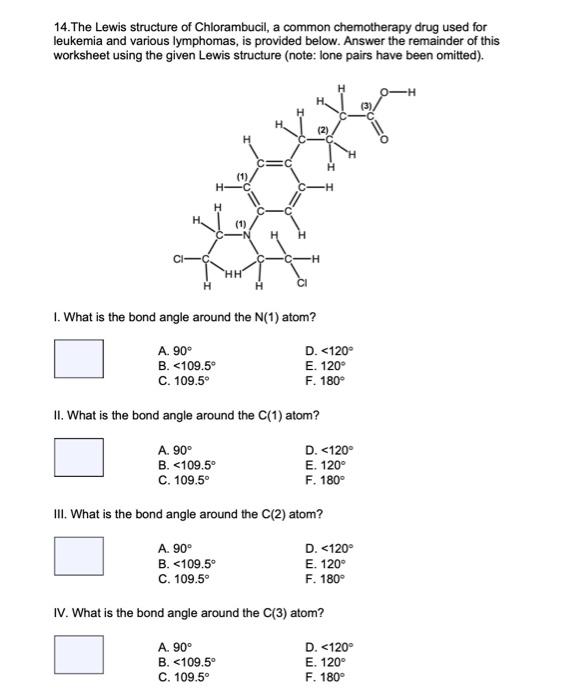
14.The Lewis structure of Chlorambucil, a common chemotherapy drug used for leukemia and various lymphomas, is provided below. Answer the remainder of this worksheet using the given Lewis structure (note: lone pairs have been omitted). I. What is the bond angle around the \( N(1) \) atom? A. \( 90^{\circ} \) D. \( <120^{\circ} \) B. \( <109.5^{\circ} \) E. \( 120^{\circ} \) C. \( 109.5^{\circ} \) F. \( 180^{\circ} \) II. What is the bond angle around the \( \mathrm{C}(1) \) atom? A. \( 90^{\circ} \) D. \( <120^{\circ} \) B. \( <109.5^{\circ} \) E. \( 120^{\circ} \) C. \( 109.5^{\circ} \) F. \( 180^{\circ} \) III. What is the bond angle around the \( \mathrm{C}(2) \) atom? A. \( 90^{\circ} \) D. \( <120^{\circ} \) B. \( <109.5^{\circ} \) E. \( 120^{\circ} \) C. \( 109.5^{\circ} \) F. \( 180^{\circ} \) IV. What is the bond angle around the \( \mathrm{C}(3) \) atom? A. \( 90^{\circ} \) D. \( <120^{\circ} \) B. \( <109.5^{\circ} \) E. \( 120^{\circ} \) C. \( 109.5^{\circ} \) F. \( 180^{\circ} \)
V. What is the hybridization around the \( \mathrm{N}(1) \) atom? A. \( s p \) D. \( s p^{3} d \) B. \( \mathrm{sp}^{2} \) E. \( s p^{3} \mathrm{~d}^{2} \) C. \( s p^{3} \) F. None of the above VI. What is the hybridization around the \( \mathrm{C}(1) \) atom? A. \( \mathrm{sp} \) B. \( \mathrm{sp}^{2} \) D. \( s p^{3} d \) C. \( s p^{3} \) E. \( s p^{3} d^{2} \) F. None of the above VII. What is the hybridization around the \( \mathrm{C}(2) \) atom? A. \( s p \) D. \( s p^{3} d \) B. \( \mathrm{sp}^{2} \) C. \( \mathrm{sp}^{3} \) F. None of the above VIII. What is the hybridization around the \( \mathrm{C}(3) \) atom? A. \( s p \) D. \( {s p^{3} d}^{3} \) B. \( \mathrm{sp}^{2} \) E. \( s p^{3} d^{2} \) C. \( \mathrm{sp}^{3} \) F. None of the above IX. What orbitals are involved in the bonding of the \( \mathrm{COOH} \) group? Select all that apply to the carbon, two oxygens, and hydrogen. A. \( s \) B. \( p \) C. \( s p \) D. \( s p^{2} \) E. \( \mathrm{sp}^{3} \) F. \( s p^{3} d \) G. \( s p^{3} d^{2} \)