Home /
Expert Answers /
Chemistry /
13-write-the-equilibrium-constant-for-the-following-reversible-reactions-3-points-for-the-first-pa237
(Solved): 13. Write the equilibrium constant for the following reversible reactions. ( 3 points for the first ...

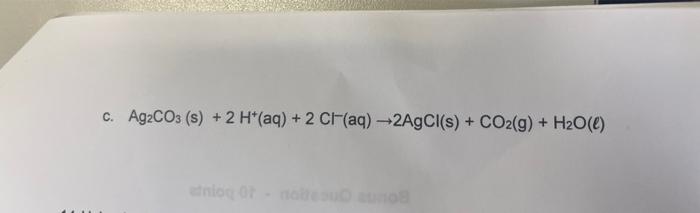
13. Write the equilibrium constant for the following reversible reactions. ( 3 points for the first two equations, 4 for the third equation) a. \( \mathrm{N}_{2}(g)+3 \mathrm{H}_{2}(g) \Leftrightarrow 2 \mathrm{NH}_{3}(g) \) b. \( \mathrm{CS}_{2}(g)+4 \mathrm{H}_{2}(g) \Leftrightarrow 2 \mathrm{H}_{2} \mathrm{~S}(g)+\mathrm{CH}_{4}(g) \)
\( \mathrm{Ag}_{2} \mathrm{CO}_{3}(\mathrm{~s})+2 \mathrm{H}^{+}(\mathrm{aq})+2 \mathrm{Cl}^{-}(\mathrm{aq}) \rightarrow 2 \mathrm{AgCl}(\mathrm{s})+\mathrm{CO}_{2}(\mathrm{~g})+\mathrm{H}_{2} \mathrm{O}(\ell) \)
Expert Answer
a) N2(g) + 3H2(g) ----------> 2NH3(g) Kc = [NH3