Home /
Expert Answers /
Chemistry /
13-lable-the-followings-on-the-gragh-3-points-i-equivalence-point-ii-half-way-point-iii-pa904
(Solved): 13. Lable the followings on the gragh ( 3 points) i. Equivalence point. ii. Half-way point iii. \( ...
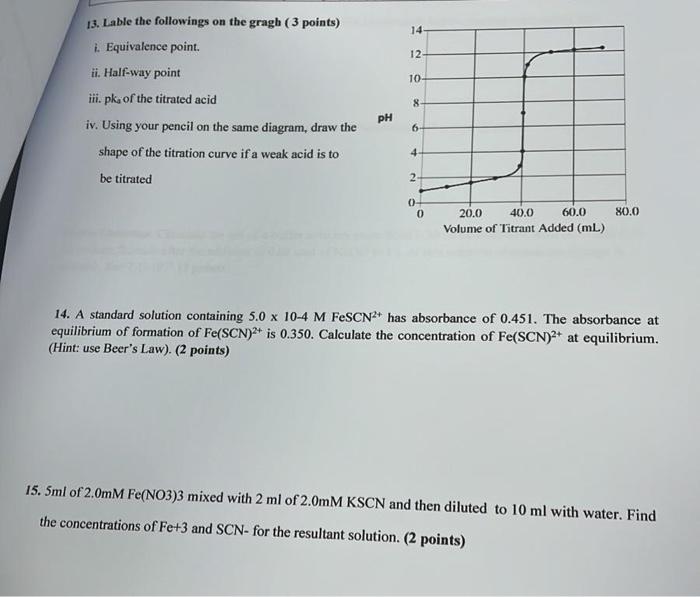
13. Lable the followings on the gragh ( 3 points) i. Equivalence point. ii. Half-way point iii. \( \mathrm{pk}_{\mathrm{a}} \) of the titrated acid iv. Using your pencil on the same diagram, draw the shape of the titration curve if a weak acid is to be titrated 14. A standard solution containing \( 5.0 \times 10-4 \mathrm{M} \mathrm{FeSCN}^{2+} \) has absorbance of \( 0.451 \). The absorbance at equilibrium of formation of \( \mathrm{Fe}(\mathrm{SCN})^{2+} \) is \( 0.350 \). Calculate the concentration of \( \mathrm{Fe}(\mathrm{SCN})^{2+} \) at equilibrium. (Hint: use Beer's Law). (2 points) 5. \( 5 \mathrm{ml} \) of \( 2.0 \mathrm{mM} \mathrm{Fe(NO3)3} \) mixed with \( 2 \mathrm{ml} \) of \( 2.0 \mathrm{mM} \mathrm{KSCN} \) and then diluted to \( 10 \mathrm{ml} \) with water. Find the concentrations of Fe+3 and \( \mathrm{SCN} \) - for the resultant solution. (2 points)
Expert Answer
14. USING A1A2=C1C2 WHERE A= ABSORBANCE AND C =CONCENTRATION 0.4510.35