Home /
Expert Answers /
Chemistry /
12-the-reaction-of-benzene-with-in-the-presence-of-anhydrous-aluminum-chloride-produces-princi-pa204
(Solved): 12. The reaction of benzene with in the presence of anhydrous aluminum chloride produces princi ...
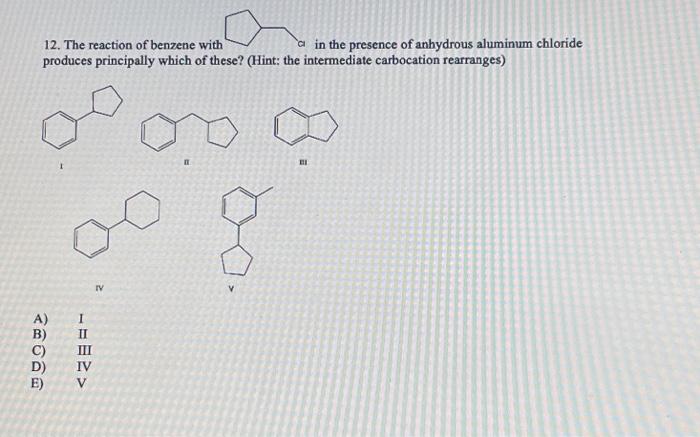
12. The reaction of benzene with in the presence of anhydrous aluminum chloride produces principally which of these? (Hint: the intermediate carbocation rearranges) n EI
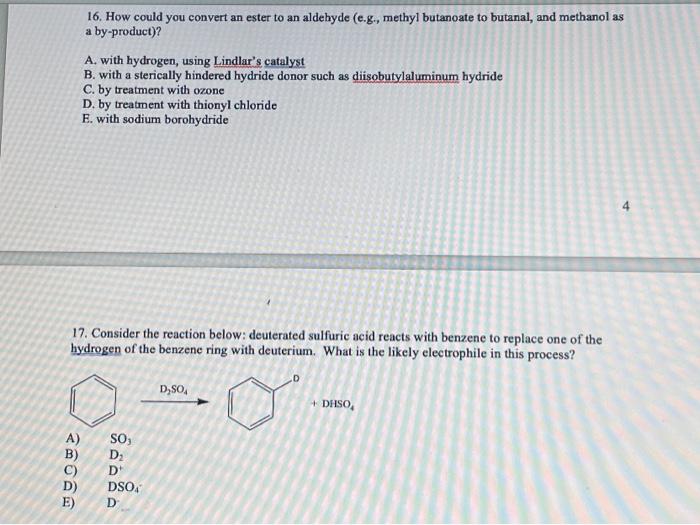
16. How could you convert an ester to an aldehyde (e.g., methyl butanoate to butanal, and methanol as a by-product)? A. with hydrogen, using Lindlar's catalyst B. with a sterically hindered hydride donor such as diisobutylaluminum hydride C. by treatment with ozone D. by treatment with thionyl chloride E. with sodium borohydride 4 17. Consider the reaction below: deuterated sulfuric acid reacts with benzene to replace one of the hydrogen of the benzene ring with deuterium. What is the likely electrophile in this process? A) B) C) D) E)
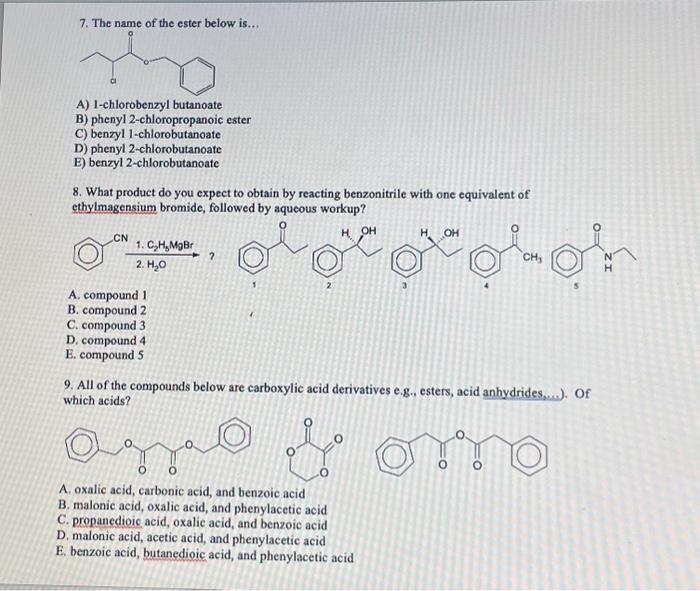
7. The name of the ester below is... A) 1-chlorobenzyl butanoate B) phenyl 2-chloropropanoic ester C) benzyl 1-chlorobutanoate D) phenyl 2-chlorobutanoate E) benzyl 2-chlorobutanoate 8. What product do you expect to obtain by reacting benzonitrile with one equivalent of ethylmagensium bromide, followed by aqueous workup? ? A. compound 1 , 2 3 4 s B. compound 2 C. compound 3 D. compound 4 E. compound 5 9. All of the compounds below are carboxylic acid derivatives e.g., esters, acid anhydrides,...). Of which acids? A. oxalic acid, carbonic acid, and benzoic acid B. malonic acid, oxalic acid, and phenylacetic acid C. propanedioic acid, oxalic acid, and benzoic acid D. malonic acid, acetic acid, and phenylacetic acid E. benzoic acid, butanedioic acid, and phenylacetic acid
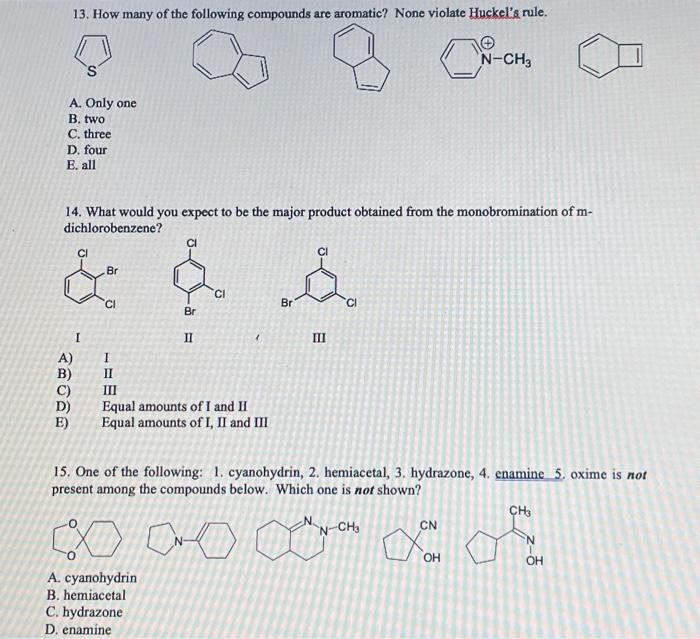
13. How many of the following compounds are aromatic? None violate Huckel's rule. A. Only one B. two C. three D. four E. all 14. What would you expect to be the major product obtained from the monobromination of dichlorobenzene? I II III A) I B) II C) III D) Equal amounts of I and II E) Equal amounts of I, II and III 15. One of the following: 1. cyanohydrin, 2. hemiacetal, 3. hydrazone, 4. enamine 5. oxime is not present among the compounds below. Which one is not shown? A. cyanohydrin B. hemiacetal C. hydrazone D. enamine
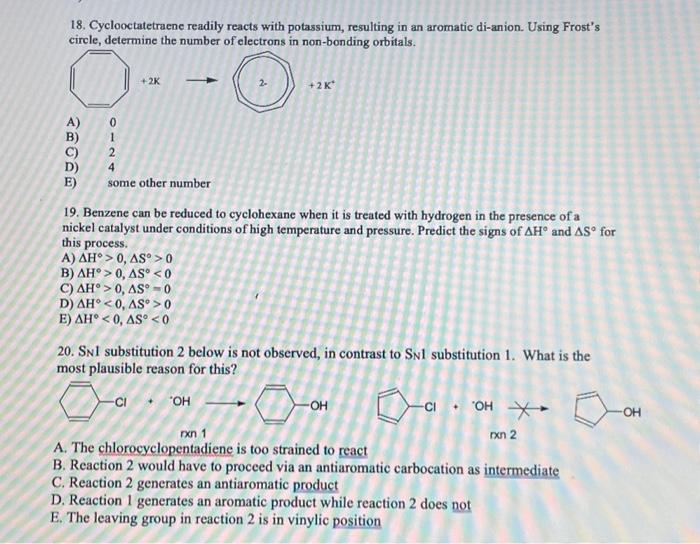
18. Cyclooctatetraene readily reacts with potassium, resulting in an aromatic di-anion. Using Frost's circle, determine the number of electrons in non-bonding orbitals. A) 0 B) 1 C) 2 D) 4 E) some other number 19. Benzene can be reduced to cyclohexane when it is treated with hydrogen in the presence of a nickel catalyst under conditions of high temperature and pressure. Predict the signs of and for this process. A) B) C) D) E) 20. substitution 2 below is not observed, in contrast to substitution 1 . What is the most plausible reason for this? A. The chlorocyclopentadiene is too strained to react B. Reaction 2 would have to proceed via an antiaromatic carbocation as intermediate C. Reaction 2 generates an antiaromatic product D. Reaction 1 generates an aromatic product while reaction 2 does not E. The leaving group in reaction 2 is in vinylic position
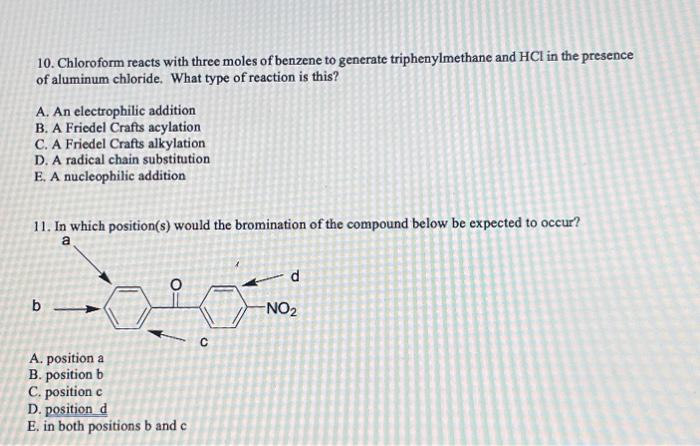
10. Chloroform reacts with three moles of benzene to generate triphenylmethane and in the presence of aluminum chloride. What type of reaction is this? A. An electrophilic addition B. A Friedel Crafts acylation C. A Friedel Crafts alkylation D. A radical chain substitution E, A nucleophilic addition 11. In which position(s) would the bromination of the compound below be expected to occur? A. position a B. position b C. position c D. position d E. in both positions and