Home /
Expert Answers /
Chemistry /
1-use-formal-charge-analysis-to-determine-which-of-the-following-lewis-structures-is-the-most-lik-pa700
(Solved): 1.) Use formal charge analysis to determine which of the following Lewis structures is the most lik ...
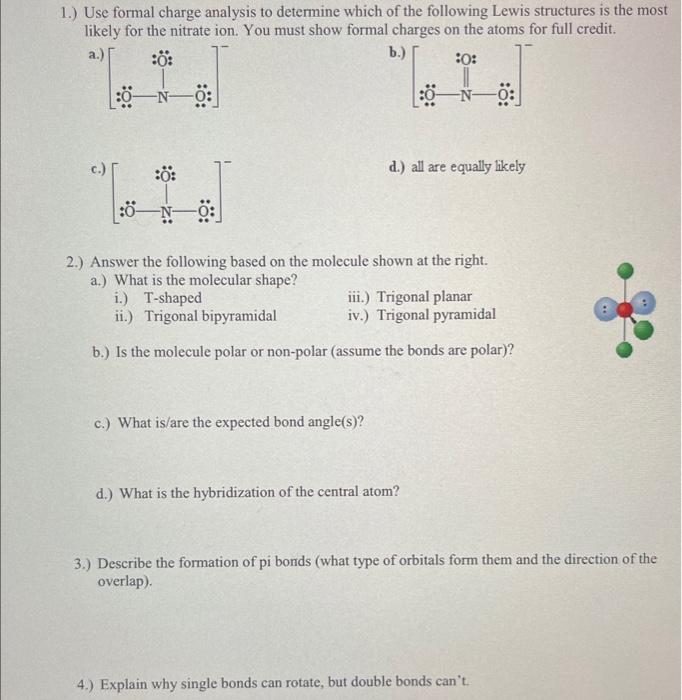
1.) Use formal charge analysis to determine which of the following Lewis structures is the most likely for the nitrate ion. You must show formal charges on the atoms for full credit. b.) a. c.) d.) all are equally likely 2.) Answer the following based on the molecule shown at the right. a.) What is the molecular shape? i.) T-shaped iii.) Trigonal planar ii.) Trigonal bipyramidal iv.) Trigonal pyramidal b.) Is the molecule polar or non-polar (assume the bonds are polar)? c.) What is/are the expected bond angle(s)? d.) What is the hybridization of the central atom? 3.) Describe the formation of pi bonds (what type of orbitals form them and the direction of the overlap). 4.) Explain why single bonds can rotate, but double bonds can't.