Home /
Expert Answers /
Chemistry /
1-the-specific-heat-of-a-metal-is-determined-by-heating-the-weighed-metal-adding-it-to-water-in-a-pa707
(Solved): 1. The specific heat of a metal is determined by heating the weighed metal, adding it to water in a ...

1. The specific heat of a metal is determined by heating the weighed metal, adding it to water in a calorimeter, and measuring the temperature change. The specific heat of water is \( 4.180 \) Joules per \( \mathrm{g} \) per \( { }^{\circ} \mathrm{C} \). The data collected are as follows: Grams of water in the calorimeter \( 41.94 \) Grams of metal \( 17.44 \) Initial temperature of metal \( 99.57 \) Initial temperature of water \( \left({ }^{\circ} \mathrm{C}\right) \quad 22.28 \) Maximum temperature of water after metal is added \( \left({ }^{\circ} \mathrm{C}\right) 23.43 \) Calculate the following: Joules gained by water (= joules lost by metal) (keep two decimal places)
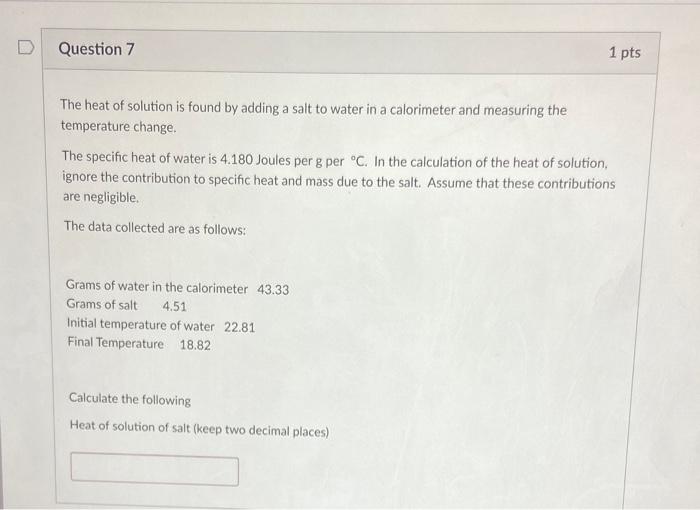
The heat of solution is found by adding a salt to water in a calorimeter and measuring the temperature change. The specific heat of water is \( 4.180 \) Joules per g per \( { }^{\circ} \mathrm{C} \). In the calculation of the heat of solution, ignore the contribution to specific heat and mass due to the salt. Assume that these contributions are negligible. The data collected are as follows: Grams of water in the calorimeter \( 43.33 \) Grams of salt \( 4.51 \) Initial temperature of water \( 22.81 \) Final Temperature \( 18.82 \) Calculate the following Heat of solution of salt (keep two decimal places)