Home /
Expert Answers /
Chemistry /
1-the-chemical-formula-of-zinc-nitrate-is-mathrm-zn-left-mathrm-no-3-right-2-a-if-pa635
(Solved): 1. The chemical formula of zinc nitrate is \( \mathrm{Zn}\left(\mathrm{NO}_{3}\right)_{2} \). a. If ...
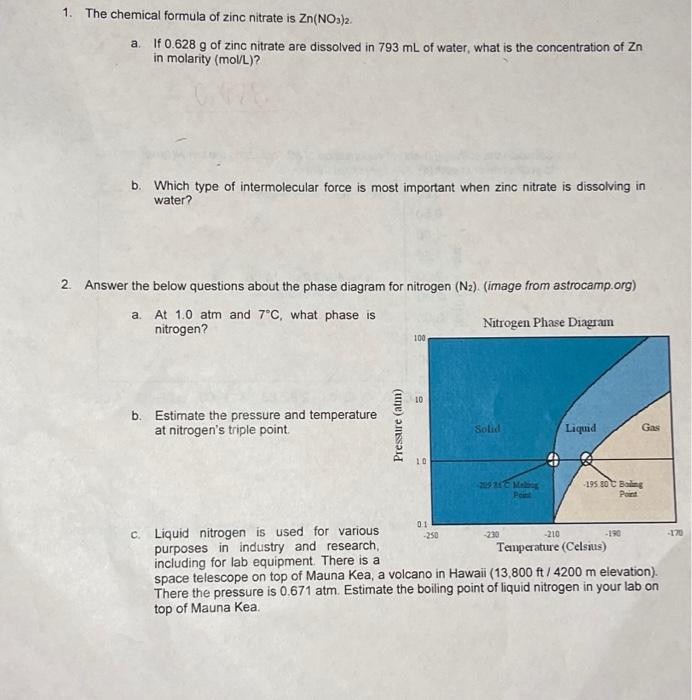
1. The chemical formula of zinc nitrate is \( \mathrm{Zn}\left(\mathrm{NO}_{3}\right)_{2} \). a. If \( 0.628 \mathrm{~g} \) of zinc nitrate are dissolved in \( 793 \mathrm{~mL} \) of water, what is the concentration of \( \mathrm{Zn} \) in molarity \( (\mathrm{mol} / \mathrm{L}) \) ? b. Which type of intermolecular force is most important when zinc nitrate is dissolving in water? 2. Answer the below questions about the phase diagram for nitrogen \( \left(\mathrm{N}_{2}\right) \). (image from astrocamp.org) a. At \( 1.0 \mathrm{~atm} \) and \( 7^{\circ} \mathrm{C} \), what phase is nitrogen? b. Estimate the pressure and temperature at nitrogen's triple point. c. Liquid nitrogen is used for various purposes in industry and research, including for lab equipment. There is a space telescope on top of Mauna Kea, a volcano in Hawaii (13,800 ft / \( 4200 \mathrm{~m} \) elevation). There the pressure is \( 0.671 \mathrm{~atm} \). Estimate the boiling point of liquid nitrogen in your lab on top of Mauna Kea.
Expert Answer
1a. The concentration of Zn in molarity is 0.0053 mol/L b. The most important intermolecular force when zinc nitrate is dissolving in water is ion-dipole interaction. Explanation: 1a. Molarity is a measure of the concentration of a solute in a soluti