Home /
Expert Answers /
Advanced Physics /
1-study-the-phenol-water-phase-diagram-below-and-answer-the-following-questions-regarding-systems-pa485
(Solved): 1. Study the phenol/water phase diagram below and answer the following questions regarding systems ...
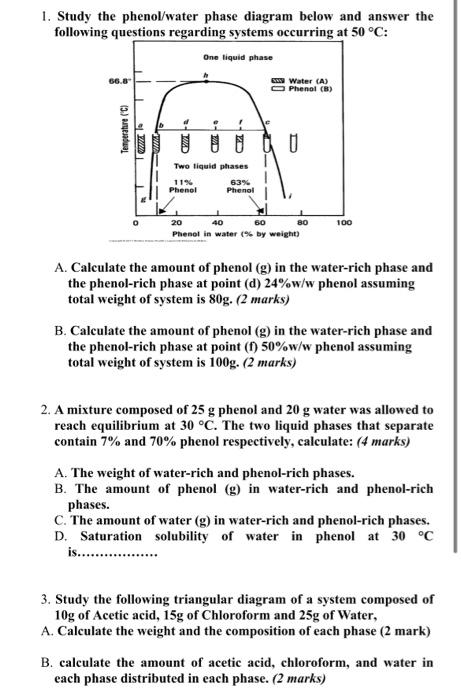
1. Study the phenol/water phase diagram below and answer the following questions regarding systems occurring at 50 °C: One liquid phase 66.8 Water (A) Phenol (B) ? Temperature (°C) Two liquid phases 11% Phenol 63% Phenol 0 20 40 60 80 100 Phenol in water (% by weight) A. Calculate the amount of phenol (g) in the water-rich phase and the phenol-rich phase at point (d) 24% w/w phenol assuming total weight of system is 80g. (2 marks) B. Calculate the amount of phenol (g) in the water-rich phase and the phenol-rich phase at point (f) 50% w/w phenol assuming total weight of system is 100g. (2 marks) 2. A mixture composed of 25 g phenol and 20 g water was allowed to reach equilibrium at 30 °C. The two liquid phases that separate contain 7% and 70% phenol respectively, calculate: (4 marks) A. The weight of water-rich and phenol-rich phases. B. The amount of phenol (g) in water-rich and phenol-rich phases. C. The amount of water (g) in water-rich and phenol-rich phases. D. Saturation solubility of water in phenol at 30 °C is............... 3. Study the following triangular diagram of a system composed of 10g of Acetic acid, 15g of Chloroform and 25g of Water, A. Calculate the weight and the composition of each phase (2 mark) B. calculate the amount of acetic acid, chloroform, and water in each phase distributed in each phase. (2 marks)
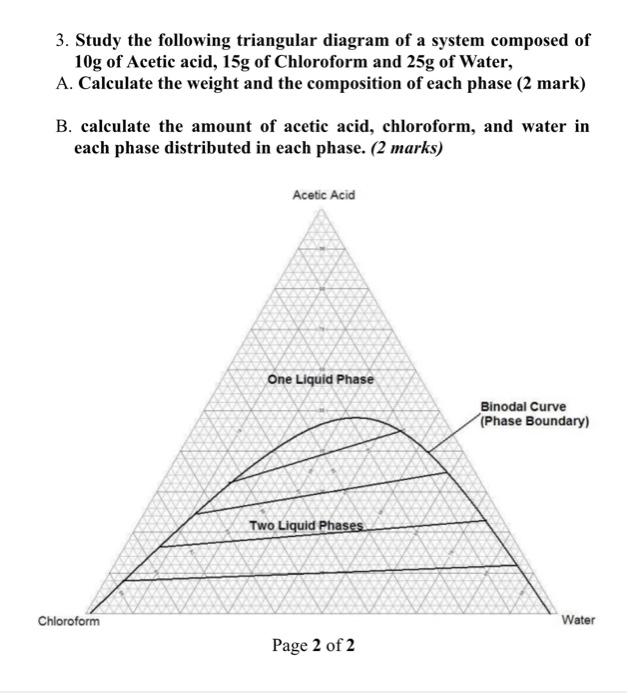
3. Study the following triangular diagram of a system composed of 10g of Acetic acid, 15g of Chloroform and 25g of Water, A. Calculate the weight and the composition of each phase (2 mark) B. calculate the amount of acetic acid, chloroform, and water in each phase distributed in each phase. (2 marks) Acetic Acid One Liquid Phase Binodal Curve (Phase Boundary) Two Liquid Phases Water Page 2 of 2 Chloroform