Home /
Expert Answers /
Chemistry /
1-2-3-the-compound-shown-below-is-the-product-of-a-claisen-condensation-draw-structural-pa489
(Solved): 1. 2. 3. The compound shown below is the product of a Claisen condensation. Draw structural ...
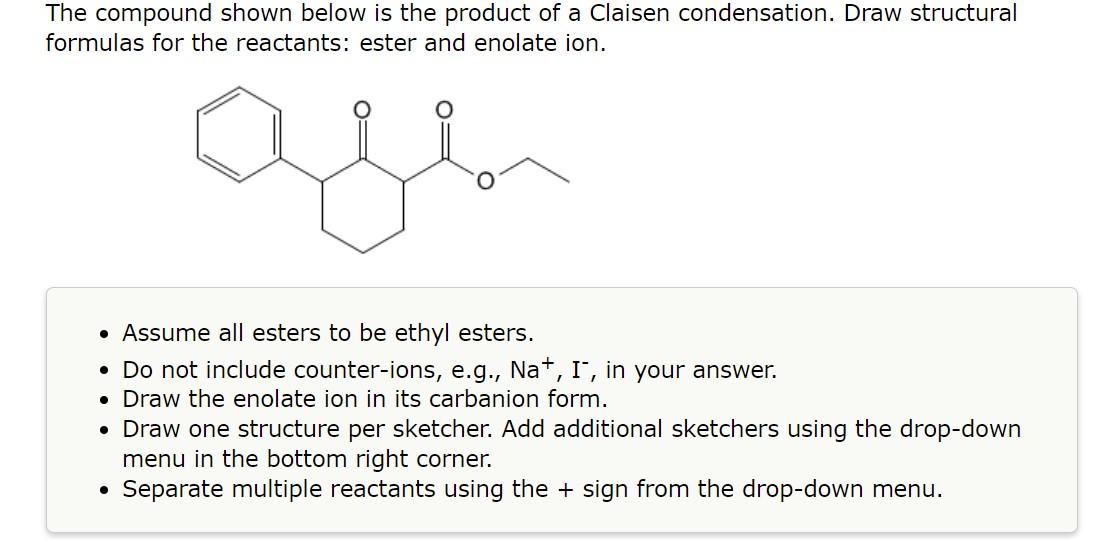
1.
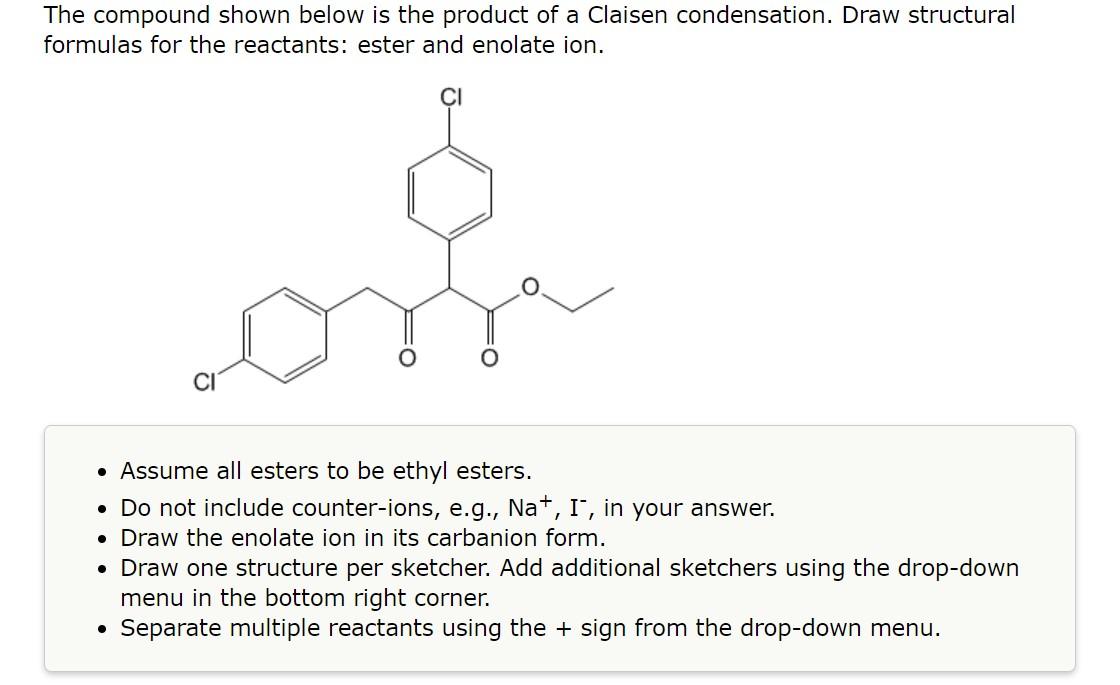
2.
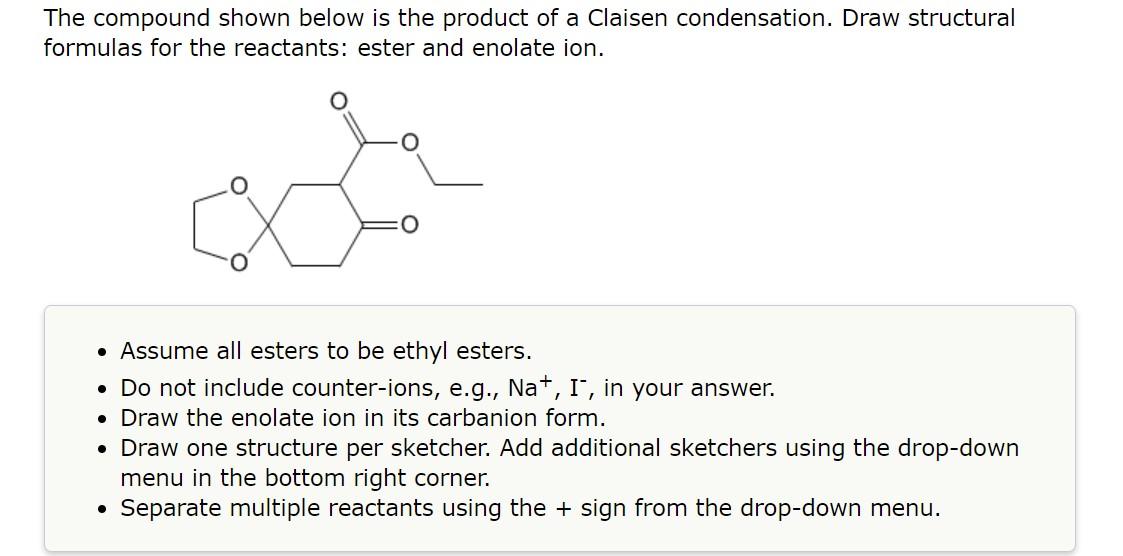
3.
The compound shown below is the product of a Claisen condensation. Draw structural formulas for the reactants: ester and enolate ion. - Assume all esters to be ethyl esters. - Do not include counter-ions, e.g., \( \mathrm{Na}^{+}, \mathrm{I}^{-} \), in your answer. - Draw the enolate ion in its carbanion form. - Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. - Separate multiple reactants using the \( + \) sign from the drop-down menu.
The compound shown below is the product of a Claisen condensation. Draw structural formulas for the reactants: ester and enolate ion. - Assume all esters to be ethyl esters. - Do not include counter-ions, e.g., \( \mathrm{Na}^{+}, \mathrm{I}^{-} \), in your answer. - Draw the enolate ion in its carbanion form. - Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. - Separate multiple reactants using the \( + \) sign from the drop-down menu.
The compound shown below is the product of a Claisen condensation. Draw structural formulas for the reactants: ester and enolate ion. - Assume all esters to be ethyl esters. - Do not include counter-ions, e.g., \( \mathrm{Na}^{+}, \mathrm{I}^{-} \), in your answer. - Draw the enolate ion in its carbanion form. - Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. - Separate multiple reactants using the \( + \) sign from the drop-down menu.